Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study
- PMID: 31829241
- PMCID: PMC6907114
- DOI: 10.1186/s12916-019-1460-0
Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study
Abstract
Background: In January 2017, the Dutch cervical cancer screening programme transitioned from cytomorphological to primary high-risk HPV (hrHPV) DNA screening, including the introduction of self-sampling, for women aged between 30 and 60 years. The Netherlands was the first country to switch to hrHPV screening at the national level. We investigated the health impact of this transition by comparing performance indicators from the new hrHPV-based programme with the previous cytology-based programme.
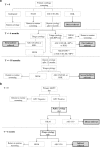
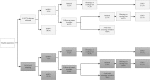
Methods: We obtained data from the Dutch nationwide network and registry of histo- and cytopathology (PALGA) for 454,573 women eligible for screening in 2017 who participated in the hrHPV-based programme between 1 January 2017 and 30 June 2018 (maximum follow-up of almost 21 months) and for 483,146 women eligible for screening in 2015 who participated in the cytology-based programme between 1 January 2015 and 31 March 2016 (maximum follow-up of 40 months). We compared indicators of participation (participation rate), referral (screen positivity; referral rate) and detection (cervical intraepithelial neoplasia (CIN) detection; number of referrals per detected CIN lesion).
Results: Participation in the hrHPV-based programme was significantly lower than that in the cytology-based programme (61% vs 64%). Screen positivity and direct referral rates were significantly higher in the hrHPV-based programme (positivity rate: 5% vs 9%; referral rate: 1% vs 3%). CIN2+ detection increased from 11 to 14 per 1000 women screened. Overall, approximately 2.2 times more clinical irrelevant findings (i.e. ≤CIN1) were found in the hrHPV-based programme, compared with approximately 1·3 times more clinically relevant findings (i.e. CIN2+); this difference was mostly due to a national policy change recommending colposcopy, rather than observation, of hrHPV-positive, ASC-US/LSIL results in the hrHPV-based programme.
Conclusions: This is the first time that comprehensive results of nationwide implementation of hrHPV-based screening have been reported using high-quality data with a long follow-up. We have shown that both benefits and potential harms are higher in one screening round of a well-implemented hrHPV-based screening programme than in an established cytology-based programme. Lower participation in the new hrHPV programme may be due to factors such as invitation policy changes and the phased roll-out of the new programme. Our findings add further to evidence from trials and modelling studies on the effectiveness of hrHPV-based screening.
Keywords: Cancer screening programmes; Cervical cancer screening; Population-based screening; hrHPV screening.
Conflict of interest statement
CA, HvA and IdK report receiving funding from the Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu) for the conduct of this study. AM reports receiving funding from the Facilitaire Samenwerking Bevolkingsonderzoeken for work related to this study and funding from DDL Laboratories outside of the study. All other authors have no conflicts of interest to declare.
Figures



References
-
- Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. - DOI - PubMed
-
- Dutch Health Council: Population screening for cervical cancer [in Dutch]. The Hague: Health Council of the Netherlands; 2011.
-
- van der Veen N, Carpay M, van Delden J, Grievink L, B. H, Lock A, Salverda J: Feasibility study for improvements to the population screening for cervical cancer [in Dutch]. Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu (RIVM); 2013.
-
- van der Veen N: Framework for the execution of cervical cancer population screening [in Dutch]. Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu (RIVM); 2017.

