Point-of-Use Detection of Environmental Fluoride via a Cell-Free Riboswitch-Based Biosensor
- PMID: 31829623
- PMCID: PMC7412506
- DOI: 10.1021/acssynbio.9b00347
Point-of-Use Detection of Environmental Fluoride via a Cell-Free Riboswitch-Based Biosensor
Abstract
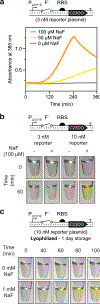
Advances in biosensor engineering have enabled the design of programmable molecular systems to detect a range of pathogens, nucleic acids, and chemicals. Here, we engineer and field-test a biosensor for fluoride, a major groundwater contaminant of global concern. The sensor consists of a cell-free system containing a DNA template that encodes a fluoride-responsive riboswitch regulating genes that produce a fluorescent or colorimetric output. Individual reactions can be lyophilized for long-term storage and detect fluoride at levels above 2 ppm, the Environmental Protection Agency's most stringent regulatory standard, in both laboratory and field conditions. Through onsite detection of fluoride in a real-world water source, this work provides a critical proof-of-principle for the future engineering of riboswitches and other biosensors to address challenges for global health and the environment.
Keywords: biosensor; cell-free systems; diagnostics; field use; riboswitches; water quality.
Figures



References
-
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals; World Health Organization, 2016.
-
- Maheshwari RC Fluoride in Drinking Water and Its Removal. J. Hazard. Mater 2006, 137 (1), 456–463. - PubMed
-
- World Health Organization. Guidelines for Drinking-Water Quality. WHO Chron. 2011, 38 (4), 104–108. - PubMed
-
- Zhou Y; Zhang JF; Yoon J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev 2014, 114 (10), 5511–5571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

