Generation of a Transcriptional Radiation Exposure Signature in Human Blood Using Long-Read Nanopore Sequencing
- PMID: 31829904
- PMCID: PMC7055010
- DOI: 10.1667/RR15476.1
Generation of a Transcriptional Radiation Exposure Signature in Human Blood Using Long-Read Nanopore Sequencing
Abstract
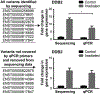
In the event of a large-scale event leading to acute ionizing radiation exposure, high-throughput methods would be required to assess individual dose estimates for triage purposes. Blood-based gene expression is a broad source of biomarkers of radiation exposure which have great potential for providing rapid dose estimates for a large population. Time is a crucial component in radiological emergencies and the shipment of blood samples to relevant laboratories presents a concern. In this study, we performed nanopore sequencing analysis to determine if the technology can be used to detect radiation-inducible genes in human peripheral blood mononuclear cells (PBMCs). The technology offers not only long-read sequencing but also a portable device which can overcome issues involving sample shipment, and provide faster results. For this goal, blood from nine healthy volunteers was 2 Gy ex vivo X irradiated. After PBMC isolation, irradiated samples were incubated along with the controls for 24 h at 37°C. RNA was extracted, poly(A)+ enriched and reverse-transcribed before sequencing. The data generated was analyzed using a Snakemake pipeline modified to handle paired samples. The sequencing analysis identified a radiation signature consisting of 46 differentially expressed genes (DEGs) which included 41 protein-coding genes, a long non-coding RNA and four pseudogenes, five of which have been identified as radiation-responsive transcripts for the first time. The genes in which transcriptional expression is most significantly modified after radiation exposure were APOBEC3H and FDXR, presenting a 25- and 28-fold change on average, respectively. These levels of transcriptional response were comparable to results we obtained by quantitative polymerase chain reaction (qPCR) analysis. In vivo exposure analyses showed a transcriptional radioresponse at 24 h postirradiation for both genes together with a strong dose-dependent response in blood irradiated ex vivo. Finally, extrapolating from the data we obtained, the minimum sequencing time required to detect an irradiated sample using APOBEC3H transcripts would be less than 3 min for a total of 50,000 reads. Future improvements, in sample processing and bioinformatic pipeline for specific radiation-responsive transcript identification, will allow the provision of a portable, rapid, real-time biodosimetry platform based on this new sequencing technology. In summary, our data show that nanopore sequencing can identify radiation-responsive genes and can also be used for identification of new transcripts.
Figures






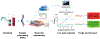
References
-
- Hall J, Jeggo PA, West C, Gomolka M, Quintens R, Badie C, et al. Ionizing radiation biomarkers in epidemiological studies – An update. Mutat Res 2017; 771:59–84. - PubMed
-
- Ainsbury E, Badie C, Barnard S, Manning G, Moquet J, Abend M, et al. Integration of new biological and physical retrospective dosimetry methods into EU emergency response plans - joint RENEB and EURADOS inter-laboratory comparisons. Int J Radiat Biol 2017; 93:99–109. - PubMed
-
- Kulka U, Abend M, Ainsbury E, Badie C, Barquinero JF, Barrios L, et al. RENEB - Running the European Network of biological dosimetry and physical retrospective dosimetry. Int J Radiat Biol 2017; 93:2–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

