Glucocorticoids counteract hypertrophic effects of myostatin inhibition in dystrophic muscle
- PMID: 31830002
- PMCID: PMC7030817
- DOI: 10.1172/jci.insight.133276
Glucocorticoids counteract hypertrophic effects of myostatin inhibition in dystrophic muscle
Abstract
Duchenne muscular dystrophy (DMD) is a devastating genetic muscle disease resulting in progressive muscle degeneration and wasting. Glucocorticoids, specifically prednisone/prednisolone and deflazacort, are commonly used by DMD patients. Emerging DMD therapeutics include those targeting the muscle-wasting factor, myostatin (Mstn). The aim of this study was to investigate how chronic glucocorticoid treatment impacts the efficacy of Mstn inhibition in the D2.mdx mouse model of DMD. We report that chronic treatment of dystrophic mice with prednisolone (Pred) causes significant muscle wasting, entailing both activation of the ubiquitin-proteasome degradation pathway and inhibition of muscle protein synthesis. Combining Pred with Mstn inhibition, using a modified Mstn propeptide (dnMstn), completely abrogates the muscle hypertrophic effects of Mstn inhibition independently of Mstn expression or SMAD3 activation. Transcriptomic analysis identified that combining Pred with dnMstn treatment affects gene expression profiles associated with inflammation, metabolism, and fibrosis. Additionally, we demonstrate that Pred-induced muscle atrophy is not prevented by Mstn ablation. Therefore, glucocorticoids interfere with potential muscle mass benefits associated with targeting Mstn, and the ramifications of glucocorticoid use should be a consideration during clinical trial design for DMD therapeutics. These results have significant implications for past and future Mstn inhibition trials in DMD.
Keywords: Monogenic diseases; Muscle; Muscle Biology; Therapeutics; Translation.
Conflict of interest statement
Figures


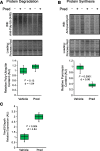

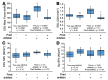


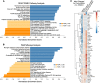
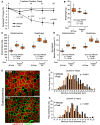
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

