Diversity and distribution of microbial communities in floral nectar of two night-blooming plants of the Sonoran Desert
- PMID: 31830071
- PMCID: PMC6907802
- DOI: 10.1371/journal.pone.0225309
Diversity and distribution of microbial communities in floral nectar of two night-blooming plants of the Sonoran Desert
Abstract
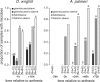
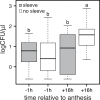
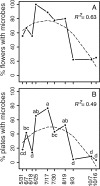
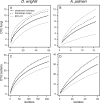
Nectar-inhabiting microbes are increasingly appreciated as important components of plant-pollinator interactions. We quantified the incidence, abundance, diversity, and composition of bacterial and fungal communities in floral nectar of two night-blooming plants of the Sonoran Desert over the course of a flowering season: Datura wrightii (Solanaceae), which is pollinated by hawkmoths, and Agave palmeri (Agavaceae), which is pollinated by bats but visited by hawkmoths that forage for nectar. We examined the relevance of growing environment (greenhouse vs. field), time (before and after anthesis), season (from early to late in the flowering season), and flower visitors (excluded via mesh sleeves or allowed to visit flowers naturally) in shaping microbial assemblages in nectar. We isolated and identified bacteria and fungi from >300 nectar samples to estimate richness and taxonomic composition. Our results show that microbes were common in D. wrightii and A. palmeri nectar in the greenhouse but more so in field environments, both before and especially after anthesis. Bacteria were isolated more frequently than fungi. The abundance of microbes in nectar of D. wrightii peaked near the middle of the flowering season. Microbes generally were more abundant as time for floral visitation increased. The composition of bacterial and especially fungal communities differed significantly between nectars of D. wrightii and A. palmeri, opening the door to future studies examining their functional roles in shaping nectar chemistry, attractiveness, and pollinator specialization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Eisikowitch D, Kevan PG, Lachance MA. The nectar-inhabiting yeasts and their effect on pollen germination in common milkweed, Asclepias syriaca L. Isr J Bot. 1990;39: 217–225.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

