Cannabidiol as a Treatment for Mood Disorders: A Systematic Review
- PMID: 31830820
- PMCID: PMC7385425
- DOI: 10.1177/0706743719895195
Cannabidiol as a Treatment for Mood Disorders: A Systematic Review
Abstract
Objective: To review the current evidence for efficacy of cannabidiol in the treatment of mood disorders.
Methods: We systematically searched PubMed, Embase, Web of Science, PsychInfo, Scielo, ClinicalTrials.gov , and The Cochrane Central Register of Controlled Trials for studies published up to July 31, 2019. The inclusion criteria were clinical trials, observational studies, or case reports evaluating the effect of pure cannabidiol or cannabidiol mixed with other cannabinoids on mood symptoms related to either mood disorders or other health conditions. The review was reported in accordance with guidelines from Preferred Reporting Items for Systematic reviews and Meta-Analyses protocol.
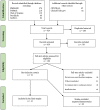
Results: Of the 924 records initially yielded by the search, 16 were included in the final sample. Among them, six were clinical studies that used cannabidiol to treat other health conditions but assessed mood symptoms as an additional outcome. Similarly, four tested cannabidiol blended with Δ-9-tetrahydrocannabinol in the treatment of general health conditions and assessed affective symptoms as secondary outcomes. Two were case reports testing cannabidiol. Four studies were observational studies that evaluated the cannabidiol use and its clinical correlates. However, there were no clinical trials investigating the efficacy of cannabidiol, specifically in mood disorders or assessing affective symptoms as the primary outcome. Although some articles point in the direction of benefits of cannabidiol to treat depressive symptoms, the methodology varied in several aspects and the level of evidence is not enough to support its indication as a treatment for mood disorders.
Conclusions: There is a lack of evidence to recommend cannabidiol as a treatment for mood disorders. However, considering the preclinical and clinical evidence related to other diseases, cannabidiol might have a role as a treatment for mood disorders. Therefore, there is an urgent need for well-designed clinical trials investigating the efficacy of cannabidiol in mood disorders.
Objectif:: Examiner les données probantes actuelles sur l’efficacité du cannabidiol dans le traitement des troubles de l’humeur.
Méthodes::
Nous avons systématiquement recherché dans PubMed, Embase, Web of Science, PsychInfo, Scielo,
Résultats:: Sur les 924 études d’abord produites par la recherche, 16 ont été incluses dans l’échantillon final. Sur celles-ci, six étaient des études cliniques qui utilisaient le cannabidiol pour traiter d’autres états de santé mais qui évaluaient les symptômes de l’humeur comme résultat additionnel. De même, quatre études testaient le cannabidiol mélangé avec du Δ-9-tétrahydrocannabinol dans le traitement d’états de santé généraux et évaluaient les symptômes affectifs comme résultats secondaires. Deux étaient des rapports de cas testant le cannabidiol. Quatre études étaient des études par observation qui évaluaient l’utilisation du cannabidiol et ses corrélats cliniques. Cependant, aucun essai clinique n’investiguait l’efficacité du cannabidiol, spécifiquement dans les troubles de l’humeur, ni n’évaluait les symptômes affectifs comme résultat principal. Bien que certains articles laissent présager les avantages du cannabidiol pour traiter les symptômes dépressifs, la méthodologie variait à plusieurs égards et le niveau des données probantes ne suffit pas à l’indiquer comme traitement des troubles de l’humeur.
Conclusions:: Les données probantes actuelles ne suffisent pas pour recommander le cannabidiol comme traitement des troubles de l’humeur. Toutefois, compte tenu des données probantes précliniques et cliniques liées à d’autres maladies, le cannabidiol pourrait jouer un rôle comme traitement des troubles de l’humeur. Il y a donc un urgent besoin d’essais cliniques bien conçus qui recherchent l’efficacité du cannabidiol dans les troubles de l’humeur.
Keywords: affective disorders; bipolar disorder; cannabidiol; cannabinoids; depressive disorders; major depressive disorder; mania; mood disorders.
Conflict of interest statement
Figures
References
-
- World Health Organization. Key facts. Department of Agriculture and Water Resources. 2018;(January):15–16.
-
- Bourne C, Aydemir O, Balanza-Martinez V, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand. 2013;128(3):149–162. - PubMed
-
- Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical