The efficacy of lyticase and β-glucosidase enzymes on biofilm degradation of Pseudomonas aeruginosa strains with different gene profiles
- PMID: 31830915
- PMCID: PMC6909625
- DOI: 10.1186/s12866-019-1662-9
The efficacy of lyticase and β-glucosidase enzymes on biofilm degradation of Pseudomonas aeruginosa strains with different gene profiles
Abstract
Background: Pseudomonas aeruginosa is a nosocomial pathogen that causes severe infections in immunocompromised patients. Biofilm plays a significant role in the resistance of this bacterium and complicates the treatment of its infections. In this study, the effect of lyticase and β-glucosidase enzymes on the degradation of biofilms of P. aeruginosa strains isolated from cystic fibrosis and burn wound infections were assessed. Moreover, the decrease of ceftazidime minimum biofilm eliminating concentrations (MBEC) after enzymatic treatment was evaluated.
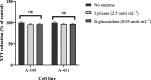
Results: This study demonstrated the effectiveness of both enzymes in degrading the biofilms of P. aeruginosa. In contrast to the lyticase enzyme, β-glucosidase reduced the ceftazidime MBECs significantly (P < 0.05). Both enzymes had no cytotoxic effect on the A-549 human lung carcinoma epithelial cell lines and A-431 human epidermoid carcinoma cell lines.
Conclusion: Considering the characteristics of the β-glucosidase enzyme, which includes the notable degradation of P. aeruginosa biofilms and a significant decrease in the ceftazidime MBECs and non-toxicity for eukaryotic cells, this enzyme can be a promising therapeutic candidate for degradation of biofilms in burn wound patients, but further studies are needed.
Keywords: Biofilm; Lyticase; Pseudomonas aeruginosa; β-Glucosidase.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Baker P, Hill PJ, Snarr BD, Alnabelseya N, Pestrak MJ, Lee MJ, Jennings LK, Tam J, Melnyk RA, Parsek MR. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci Adv. 2016;2(5):e1501632. doi: 10.1126/sciadv.1501632. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

