TRPV1 activity and substance P release are required for corneal cold nociception
- PMID: 31831729
- PMCID: PMC6908618
- DOI: 10.1038/s41467-019-13536-0
TRPV1 activity and substance P release are required for corneal cold nociception
Abstract
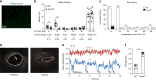
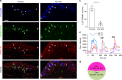
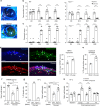
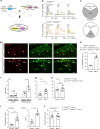
As a protective mechanism, the cornea is sensitive to noxious stimuli. Here, we show that in mice, a high proportion of corneal TRPM8+ cold-sensing fibers express the heat-sensitive TRPV1 channel. Despite its insensitivity to cold, TRPV1 enhances membrane potential changes and electrical firing of TRPM8+ neurons in response to cold stimulation. This elevated neuronal excitability leads to augmented ocular cold nociception in mice. In a model of dry eye disease, the expression of TRPV1 in TRPM8+ cold-sensing fibers is increased, and results in severe cold allodynia. Overexpression of TRPV1 in TRPM8+ sensory neurons leads to cold allodynia in both corneal and non-corneal tissues without affecting their thermal sensitivity. TRPV1-dependent neuronal sensitization facilitates the release of the neuropeptide substance P from TRPM8+ cold-sensing neurons to signal nociception in response to cold. Our study identifies a mechanism underlying corneal cold nociception and suggests a potential target for the treatment of ocular pain.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol. Vis. Sci. 2001;42:2063–2067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM101218/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)/International
- R01DK103901/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)/International
- R01 AA027065/AA/NIAAA NIH HHS/United States
- R01 AI125743/AI/NIAID NIH HHS/United States
- R01 EY024704/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

