A novel application of Gini coefficient for the quantitative measurement of bacterial aggregation
- PMID: 31831832
- PMCID: PMC6908595
- DOI: 10.1038/s41598-019-55567-z
A novel application of Gini coefficient for the quantitative measurement of bacterial aggregation
Abstract
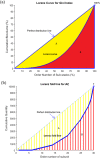
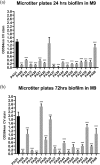
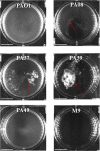
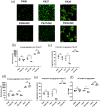
Non-surface attached bacterial aggregates are frequently found in clinical settings associated with chronic infections. Current methods quantifying the extent to which a suspended bacterial population is aggregated mainly rely on: (1) cell size distribution curves that are difficult to be compared numerically among large-scale samples; (2) the average size/proportion of aggregates in a population that do not specify the aggregation patterns. Here we introduce a novel application of Gini coefficient, herein named Aggregation Coefficient (AC), to quantify the aggregation levels of cystic fibrosis Pseudomonas aeruginosa (CF-PA) isolates in vitro using 3D micrographs, Fiji and MATLAB. Different aggregation patterns of five strains were compared statistically using the numerical AC indexes, which correlated well with the size distribution curves plotted by different biovolumes of aggregates. To test the sensitivity of AC, aggregates of the same strains were treated with nitric oxide (NO), a dispersal agent that reduces the biomass of surface attached biofilms. Strains unresponsive to NO were reflected by comparable AC indexes, while those undergoing dispersal showed a significant reduction in AC index, mirroring the changes in average aggregate sizes and proportions. Therefore, AC provides simpler and more descriptive numerical outputs for measuring different aggregation patterns compared to current approaches.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

