Toxoplasma ROP16I/III ameliorated inflammatory bowel diseases via inducing M2 phenotype of macrophages
- PMID: 31832003
- PMCID: PMC6906210
- DOI: 10.3748/wjg.v25.i45.6634
Toxoplasma ROP16I/III ameliorated inflammatory bowel diseases via inducing M2 phenotype of macrophages
Abstract
Background: Inflammatory bowel disease (IBD) is characterized by chronic and non-specific inflammation of the intestinal mucosa and mainly includes ulcerative colitis and Crohn's disease.
Aim: To explore the beneficial effect of ToxoROP16I/III-induced M2 phynotype macrophages in homeostasis of IBDs through downregulation of M1 inflammatory cells.
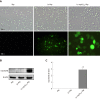
Methods: RAW264.7 macrophages stimulated by lipopolysaccharide (LPS) (M1 cells) were co-cultured with Caco-2 cells as an inflammatory model of IBD in vitro. The expression of ToxoROP16I/III was observed in RAW264.7 macrophages that were transfected with pEGFP-rop16 I/III. The phenotypes of M2 and M1 macrophage cells were assessed by quantitative real-time reverse transcriptase polymerase chain reaction and the expression of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, transforming growth factor (TGF)-β1, IL-10, inducible nitric oxide synthase (iNOS), and arginase-1 (Arg-1) was detected. The expression of iNOS, Arg-1, signal transducer and activator of transcription 3 (Stat3), p-Stat3, Stat6, p-Stat6, programmed death ligand-2 (PD-L2), caspase-3, -8, and -9 was analyzed by Western blotting, and Griess assays were performed to detect nitric oxide (NO). TNF-α, IL-1β, IL-6, TGF-β1, and IL-10 expression in the supernatants was detected by enzyme-linked immunosorbent assay, and Caco-2 cell apoptosis was determined by flow cytometry after mixing M1 cells with M2 cells in a Caco-2 cell co-culture system.
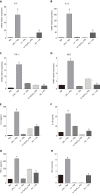
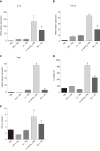
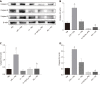
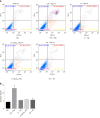
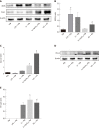
Results: M1 cells exhibited significantly increased production of iNOS, NO, TNF-α, IL-1β, and IL-6, while ToxoROP16I/III induced macrophage bias to M2 cells in vitro, showing increased expression of Arg-1, IL-10 and TGF-β1 and elevated production of p-Stat3 and p-Stat6. The mixed M1 and M2 cell culture induced by ToxoROP16I/III exhibited decreased production of NO and iNOS and upregulated expression of Arg-1 and PD-L2. Accordingly, Caco-2 cells became apoptotic, and apoptosis-associated proteins such as caspase-3, -8 and -9 were dampened during co-culture of M1 and M2 cells. Flow cytometry analysis showed that co-culture of M1 cells with Caco-2 cells facilitated the apoptosis of Caco-2 cells, but co-culture of M1 and M2 cells alleviated Caco-2 cell apoptosis.
Conclusion: ToxoROP16I/III-induced M2 macrophages inhibited apoptosis of Caco-2 cells caused by M1 macrophages. This finding may help gain a better understanding of the underlying mechanism and represent a promising therapeutic strategy for IBDs.
Keywords: Alternatively activated macrophages; Caco-2; Classically activated macrophages; Immunity; Inflammatory bowel disease; Toxoplasma ROP16I/III.
©The Author(s) 2019. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: None of the authors has any conflicts of interest to declare.
Figures


References
-
- Markowitz J, Grancher K, Kohn N, Daum F. Immunomodulatory therapy for pediatric inflammatory bowel disease: changing patterns of use, 1990-2000. Am J Gastroenterol. 2002;97:928–932. - PubMed
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. - PubMed
-
- Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. - PubMed
-
- Kim JK, Jun JG. Ailanthoidol suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J Cell Biochem. 2011;112:3816–3823. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

