Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots
- PMID: 31832313
- PMCID: PMC6891914
- DOI: 10.1002/advs.201901316
Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots
Abstract
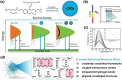
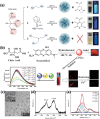
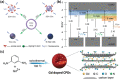
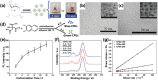
Despite the various synthesis methods to obtain carbon dots (CDs), the bottom-up methods are still the most widely administrated route to afford large-scale and low-cost synthesis. However, as CDs are developed with increasing reports involved in producing many CDs, the structure and property features have changed enormously compared with the first generation of CDs, raising classification concerns. To this end, a new classification of CDs, named carbonized polymer dots (CPDs), is summarized according to the analysis of structure and property features. Here, CPDs are revealed as an emerging class of CDs with distinctive polymer/carbon hybrid structures and properties. Furthermore, deep insights into the effects of synthesis on the structure/property features of CDs are provided. Herein, the synthesis methods of CDs are also summarized in detail, and the effects of synthesis conditions of the bottom-up methods in terms of the structures and properties of CPDs are discussed and analyzed comprehensively. Insights into formation process and nucleation mechanism of CPDs are also offered. Finally, a perspective of the future development of CDs is proposed with critical insights into facilitating their potential in various application fields.
Keywords: carbon dots; carbonized polymer dots; formation mechanism; photoluminescence mechanism; synthesis.
© 2019 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Advances in Shell and Core Engineering of Carbonized Polymer Dots for Enhanced Applications.Acc Chem Res. 2024 Oct 1;57(19):2928-2939. doi: 10.1021/acs.accounts.4c00516. Epub 2024 Sep 19. Acc Chem Res. 2024. PMID: 39298332
-
The Formation Mechanism of Carbonized Polymer Dots: Crosslinking-Induced Nucleation and Carbonization.Angew Chem Int Ed Engl. 2024 Oct 24;63(44):e202410519. doi: 10.1002/anie.202410519. Epub 2024 Sep 17. Angew Chem Int Ed Engl. 2024. PMID: 39090074
-
Precursor-dependent structural diversity in luminescent carbonized polymer dots (CPDs): the nomenclature.Light Sci Appl. 2021 Jul 12;10(1):142. doi: 10.1038/s41377-021-00579-6. Light Sci Appl. 2021. PMID: 34253707 Free PMC article.
-
The Transformation of 0-D Carbon Dots into 1-, 2- and 3-D Carbon Allotropes: A Minireview.Nanomaterials (Basel). 2022 Jul 22;12(15):2515. doi: 10.3390/nano12152515. Nanomaterials (Basel). 2022. PMID: 35893483 Free PMC article. Review.
-
Carbon dots: Types, preparation, and their boosted antibacterial activity by photoactivation. Current status and future perspectives.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023 Jul-Aug;15(4):e1887. doi: 10.1002/wnan.1887. Epub 2023 Apr 26. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023. PMID: 37100045 Review.
Cited by
-
Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots.Nanomaterials (Basel). 2022 Nov 18;12(22):4070. doi: 10.3390/nano12224070. Nanomaterials (Basel). 2022. PMID: 36432356 Free PMC article.
-
Biowaste-Derived Carbon Dots: A Perspective on Biomedical Potentials.Molecules. 2022 Sep 21;27(19):6186. doi: 10.3390/molecules27196186. Molecules. 2022. PMID: 36234727 Free PMC article. Review.
-
Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation.Molecules. 2022 Oct 2;27(19):6517. doi: 10.3390/molecules27196517. Molecules. 2022. PMID: 36235054 Free PMC article.
-
Nanomaterial application in bio/sensors for the detection of infectious diseases.Talanta. 2021 Aug 1;230:122026. doi: 10.1016/j.talanta.2020.122026. Epub 2020 Dec 17. Talanta. 2021. PMID: 33934756 Free PMC article. Review.
-
Carbon Dots-Types, Obtaining and Application in Biotechnology and Food Technology.Int J Mol Sci. 2023 Oct 7;24(19):14984. doi: 10.3390/ijms241914984. Int J Mol Sci. 2023. PMID: 37834430 Free PMC article. Review.
References
-
- a) Dai X., Deng Y., Peng X., Jin Y., Adv. Mater. 2017, 29, 1607022; - PubMed
- b) Hildebrandt N., Spillmann C. M., Algar W. R., Pons T., Stewart M. H., Oh E., Susumu K., Diaz S. A., Delehanty J. B., Medintz I. L., Chem. Rev. 2017, 117, 536; - PubMed
- c) Kagan C. R., Lifshitz E., Sargent E. H., Talapin D. V., Science 2016, 353, aac5523; - PubMed
- d) Li X., Wu Y., Zhang S., Cai B., Gu Y., Song J., Zeng H., Adv. Funct. Mater. 2016, 26, 2435;
- e) Reiss P., Carriere M., Lincheneau C., Vaure L., Tamang S., Chem. Rev. 2016, 116, 10731. - PubMed
-
- a) Baker S. N., Baker G. A., Angew. Chem., Int. Ed. 2010, 49, 6726; - PubMed
- b) Bartelmess J., Quinn S. J., Giordani S., Chem. Soc. Rev. 2015, 44, 4672; - PubMed
- c) Lim S. Y., Shen W., Gao Z., Chem. Soc. Rev. 2015, 44, 362; - PubMed
- d) Xu X. Y., Ray R., Gu Y. L., Ploehn H. J., Gearheart L., Raker K., Scrivens W. A., J. Am. Chem. Soc. 2004, 126, 12736. - PubMed
-
- a) Chakraborty I., Pradeep T., Chem. Rev. 2017, 117, 8208; - PubMed
- b) Shang L., Dong S., Nienhaus G. U., Nano Today 2011, 6, 401.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
