Benzoxazines as new human topoisomerase I inhibitors and potential poisons
- PMID: 31832989
- PMCID: PMC7214584
- DOI: 10.1007/s40199-019-00315-x
Benzoxazines as new human topoisomerase I inhibitors and potential poisons
Abstract
Background: The numbers of topoisomerase I targeted drugs on the market are very limited although they are used clinically for treatment of solid tumors. Hence, studies about finding new chemical structures which specifically target topoisomerase I are still remarkable.
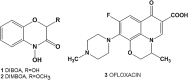
Objectives: In this present study, we tested previously synthesized 3,4-dihydro-2H-1,4-benzoxazin-3-one derivatives to reveal their human DNA topoisomerase I inhibitory potentials.
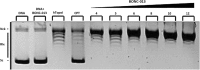
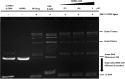
Methods: We investigated inhibitory activities of 3,4-dihydro-2H-1,4-benzoxazin-3-one derivatives on human topoisomerase I by relaxation assay to clarify inhibition mechanisms of effective derivatives with EMSA and T4 DNA ligase based intercalation assay. With SAR study, it was tried to find out effective groups in the ring system.
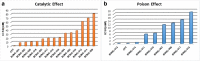
Results: While 10 compounds showed catalytic inhibitory activity, 8 compounds were found to be potential topoisomerase poisons. 4 of them also exhibited both activities. 2-hydroxy-3,4-dihydro-2H-1,4-benzoxazin-3-one (BONC-001) was the most effective catalytic inhibitor (IC50:8.34 mM) and ethyl 6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-acetate (BONC-013) was the strongest potential poison (IC50:0.0006 mM). BONC-013 was much more poisonous than camptothecin (IC50:0.034 mM). Intercalation assay showed that BONC-013 was not an intercalator and BONC-001 most probably prevented enzyme-substrate binding in an unknown way. Another important result of this study was that OH group instead of ethoxycarbonylmethyl group at R position of benzoxazine ring was important for hTopo I catalytic inhibition while the attachment of a methyl group of R1 position at R2 position were play a role for increasing of its poisonous effect.
Conclusion: As a result, we presented new DNA topoisomerase I inhibitors which might serve novel constructs for future anticancer agent designs. Graphical abstract.
Keywords: Anticancer; Benzoxazine; Catalytic inhibitor; Topoisomerase I; Topoisomerase I poison.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures


References
-
- Pfister TD, Reinhold WC, Agama K, Gupta S, Khin SA, Kinders RJ, Parchment RE, Tomaszewski JE, Doroshow JH, Pommier Y. Topoisomerase I levels in the NCI-60 cancer cell line panel determined by validated ELISA and microarray analysis and correlation with indenoisoquinoline sensitivity. Mol Cancer Ther. 2009;8(7):1878–1884. doi: 10.1158/1535-7163.MCT-09-0016. - DOI - PMC - PubMed
-
- Gouveris P, Skopelitis E, Tsavaris N. Topoisomerase I and II expression in recurrent colorectal cancer cells: a dubious matter. In: Seligmann H, editor. DNA replication-current advances. Rijeka, Croatia: InTech; 2011. pp. 639–668.
-
- Lv C, Liu X, Zheng Q, Chen H, Yang X, Zhong J, Wang Y, Duan J, Wang Z, Bai H, Wu M, Zhao J, Wang J, Wang Z, An T, Zhuo M. Analysis of topoisomerase I expression and identification of predictive markers for efficacy of topotecan chemotherapy in small cell lung cancer. Thorac Cancer. 2018;9(9):1166–1173. doi: 10.1111/1759-7714.12819. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

