Molecular and physiological consequences of faulty eukaryotic ribonucleotide excision repair
- PMID: 31833079
- PMCID: PMC6996501
- DOI: 10.15252/embj.2019102309
Molecular and physiological consequences of faulty eukaryotic ribonucleotide excision repair
Abstract
The duplication of the eukaryotic genome is an intricate process that has to be tightly safe-guarded. One of the most frequently occurring errors during DNA synthesis is the mis-insertion of a ribonucleotide instead of a deoxyribonucleotide. Ribonucleotide excision repair (RER) is initiated by RNase H2 and results in error-free removal of such mis-incorporated ribonucleotides. If left unrepaired, DNA-embedded ribonucleotides result in a variety of alterations within chromosomal DNA, which ultimately lead to genome instability. Here, we review how genomic ribonucleotides lead to chromosomal aberrations and discuss how the tight regulation of RER timing may be important for preventing unwanted DNA damage. We describe the structural impact of unrepaired ribonucleotides on DNA and chromatin and comment on the potential consequences for cellular fitness. In the context of the molecular mechanisms associated with faulty RER, we have placed an emphasis on how and why increased levels of genomic ribonucleotides are associated with severe autoimmune syndromes, neuropathology, and cancer. In addition, we discuss therapeutic directions that could be followed for pathologies associated with defective removal of ribonucleotides from double-stranded DNA.
Keywords: DNA repair; RNA-DNA hybrid; RNase H2; ribonucleotide excision repair; topoisomerase 1.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
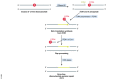
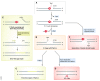
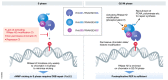

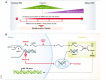

References
-
- Ahel I, Rass U, El‐Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC (2006) The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature 443: 713–716 - PubMed
-
- Akwa Y, Hassett DE, Eloranta M‐L, Sandberg K, Masliah E, Powell H, Whitton JL, Bloom FE, Campbell IL (1998) Transgenic expression of IFN‐α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol 161: 5016–5026 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

