Endocrine Disruption and Reproductive Pathology
- PMID: 31833458
- PMCID: PMC8008741
- DOI: 10.1177/0192623319879903
Endocrine Disruption and Reproductive Pathology
Abstract
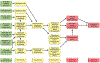
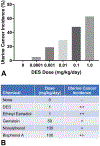
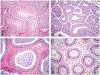
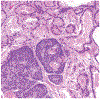
During the past 20 years, investigations involving endocrine active substances (EAS) and reproductive toxicity have dominated the landscape of ecotoxicological research. This has occurred in concert with heightened awareness in the scientific community, general public, and governmental entities of the potential consequences of chemical perturbation in humans and wildlife. The exponential growth of experimentation in this field is fueled by our expanding knowledge into the complex nature of endocrine systems and the intricacy of their interactions with xenobiotic agents. Complicating factors include the ever-increasing number of novel receptors and alternate mechanistic pathways that have come to light, effects of chemical mixtures in the environment versus those of single EAS laboratory exposures, the challenge of differentiating endocrine disruption from direct cytotoxicity, and the potential for transgenerational effects. Although initially concerned with EAS effects chiefly in the thyroid glands and reproductive organs, it is now recognized that anthropomorphic substances may also adversely affect the nervous and immune systems via hormonal mechanisms and play substantial roles in metabolic diseases, such as type 2 diabetes and obesity.
Keywords: endocrine disrupters; environmental toxicology; fish pathology; hormonal carcinogenesis; nonhuman primate; reproductive system; rodent pathology.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures










References
-
- Macleod DJ, Sharpe RM, Welsh M, et al. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33(2):279–287. - PubMed
-
- Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE JR. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008;31(2):178–187. - PubMed
-
- Blystone CR, Lambright CS, Howdeshell KL, et al. Sensitivity of fetal rat testicular steroidogenesis to maternal prochloraz exposure and the underlying mechanism of inhibition. Toxicol Sci. 2007; 97(2):512–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

