Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials
- PMID: 31834000
- PMCID: PMC7043729
- DOI: 10.1097/QAI.0000000000002275
Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials
Erratum in
-
Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials: Erratum.J Acquir Immune Defic Syndr. 2020 Jul 1;84(3):e21. doi: 10.1097/QAI.0000000000002394. J Acquir Immune Defic Syndr. 2020. PMID: 32530908 Free PMC article.
Abstract
Background: The 2-drug regimen dolutegravir + lamivudine was noninferior to dolutegravir + tenofovir disoproxil fumarate/emtricitabine in achieving HIV-1 RNA <50 copies/mL in treatment-naive adults in the 48-week primary analysis of the GEMINI trials. We present results from the prespecified 96-week secondary analyses.
Setting: One hundred eighty-seven centers in 21 countries.
Methods: GEMINI-1 and GEMINI-2 are identical, double-blind phase III studies. Participants with screening HIV-1 RNA ≤500,000 copies/mL were randomized 1:1 to once-daily dolutegravir + lamivudine or dolutegravir + tenofovir disoproxil fumarate/emtricitabine.
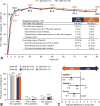
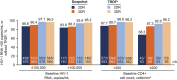
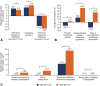
Results: At week 96, dolutegravir + lamivudine (N = 716) was noninferior to dolutegravir + tenofovir disoproxil fumarate/emtricitabine (N = 717) in achieving HIV-1 RNA <50 copies/mL (Snapshot algorithm; -10% noninferiority margin) in the pooled analysis (proportion of responders, 86.0% vs 89.5%, respectively; adjusted treatment difference [95% CI], -3.4% [-6.7 to 0.0007]), GEMINI-1 (-4.9% [-9.8 to 0.03]), and GEMINI-2 (-1.8% [-6.4 to 2.7]). Proportions of participants in the HIV-1 RNA ≥50 copies/mL Snapshot category were largely unchanged from week 48 to 96. Eleven participants taking dolutegravir + lamivudine and 7 taking dolutegravir + tenofovir disoproxil fumarate/emtricitabine met confirmed virologic withdrawal criteria through week 96; none had treatment-emergent resistance mutations. Dolutegravir + lamivudine had a lower rate of drug-related adverse events than dolutegravir + tenofovir disoproxil fumarate/emtricitabine (19.6% vs 25.0%; relative risk ratio, 0.78; 95% CI: 0.64 to 0.95). Renal and bone biomarker changes favored dolutegravir + lamivudine.
Conclusions: Consistent with 48-week data, dolutegravir + lamivudine demonstrated long-term, noninferior efficacy vs dolutegravir + tenofovir disoproxil fumarate/emtricitabine without increased risk of treatment-emergent resistance, supporting its use in treatment-naive HIV-1-infected individuals.
Conflict of interest statement
P.C. has served on advisory boards for GlaxoSmithKline (GSK), ViiV Healthcare, and Merck; served as an investigator for Abbott, Gilead, ViiV Healthcare, GSK, Merck, and Richmond; and has received honoraria for his speaking or chairing engagements from Abbott, GSK, Gilead, Merck, and ViiV Healthcare. J.S.M. has received lecture fees, sponsorship, and honoraria from Gilead, Stendhal, AbbVie, ViiV Healthcare, Janssen, and Merck Sharp & Dohme (MSD; all before 2019). J.R.A. has received advisory fees, speaker fees, and grant support from ViiV Healthcare, Janssen, Gilead, MSD, Alexa, and Teva. A.A. has served as a paid consultant to Gilead, Janssen, Merck, and ViiV Healthcare and received research funding from Gilead, Janssen, and ViiV Healthcare. A.E.C. has received advisory fees from GSK, ViiV Healthcare, and Gilead; conference sponsorship from Gilead and Janssen; and speaker travel fees from GSK. C.-C.H. has received honoraria for speaking at educational events or consulting from AbbVie, Bristol-Myers Squibb (BMS), Gilead, Janssen, and ViiV Healthcare and has received research funding from BMS, Janssen, Gilead, Merck, and ViiV Healthcare. J.K.R. has received grant/research support from Gilead; served as a consultant/advisor to Abbott, AbbVie, Bionor, Gilead, Hexal, Janssen, Merck, and ViiV Healthcare; and was a speaker at educational events for AbbVie, Gilead, Janssen, and Merck. P.-M.G. has received grants from BMS and Janssen and has received honoraria and consulting fees from Gilead, ViiV Healthcare, and AbbVie. J.S., C.Y.M., M.U., A.R.T., K.A.P., B.W., M.G., M.A., J.v.W., and K.Y.S. are employees of ViiV Healthcare and own stock in GSK. R.U. and D.B. are employees of GSK and own stock in GSK. The remaining author has no conflicts of interest to disclose.
Figures
References
-
- Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792. - PubMed
-
- Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572–580. - PubMed
-
- Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir + lamivudine versus maintenance of atazanavir/ritonavir + two NRTIs in virologically suppressed HIV-1-infected patients: 48 week results from a randomized trial (ATLAS-M). J Antimicrob Chemother. 2017;72:1163–1171. - PubMed
-
- Raffi F, Babiker AG, Richert L, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384:1942–1951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

