Interruption of Jasmonic Acid Biosynthesis Causes Differential Responses in the Roots and Shoots of Maize Seedlings against Salt Stress
- PMID: 31835299
- PMCID: PMC6969903
- DOI: 10.3390/ijms20246202
Interruption of Jasmonic Acid Biosynthesis Causes Differential Responses in the Roots and Shoots of Maize Seedlings against Salt Stress
Abstract
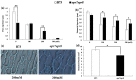
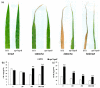
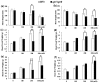
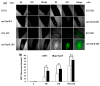
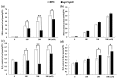
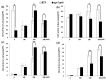
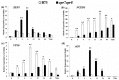
Jasmonates (JAs) together with jasmonic acid and its offshoots are lipid-derived endogenous hormones that play key roles in both developmental processes and different defense responses in plants. JAs have been studied intensively in the past decades for their substantial roles in plant defense comebacks against diverse environmental stresses among model plants. However, the role of this phytohormone has been poorly investigated in the monocotyledonous species against abiotic stresses. In this study, a JA biosynthesis mutant opr7opr8 was used for the investigation of JA roles in the salt stress responses of maize seedlings, whose roots were exposed to 0 to 300 mM NaCl. Foliar stomatal observation showed that opr7opr8 had a larger stomatal aperture than wild type (WT) (B73) under salinity stress, indicating that JA positively regulates guard cell movement under salt stress. The results regarding chlorophyll content and leaf senescence showed that opr7opr8 exhibited delayed leaf senescence under salt stress as compared to WT, indicating that JA plays a role in salt-inducing cell death and subsequent leaf senescence. Moreover, the morphological parameters, including the length of the shoots and roots, and the fresh and dry weights of the shoots and roots, showed that after 7 days of salt treatment, opr7opr8 had heavier and longer shoots than WT but slighter and shorter roots than WT. In addition, ion analysis showed that opr7opr8 accumulated less sodium but more potassium in the leaves than WT but more sodium and less potassium in the roots than WT, suggesting that JA deficiency causes higher salt stress to the roots but less stress to the leaves of the seedlings. Reactive oxygen species (ROS) analysis showed that opr7opr8 produced less H2O2 than WT in the leaves but more H2O2 in the roots under salt treatment, and correspondingly, ROS-scavenging enzymes superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) showed a similar variation, i.e., opr7opr8 has lower enzymatic activities in the shoots but higher activities in the roots than WT under salt treatment. For osmotic adjustment, opr7opr8 produced less proline in the shoots at 100 and 300 mM NaCl treatments but more in the roots than the WT roots under all salt treatments. In addition, the gene expression for abscisic acid (ABA) biosynthesis under salt stress was investigated. Results showed that the expression levels of four key enzymes of ABA biosynthesis, ZEP1, NCED5, AO1, and VP10, were significantly downregulated in the shoots as compared to WT under salt treatment. Putting all the data together, we concluded that JA-deficiency in maize seedlings reduced the salt-stress responses in the shoots but exaggerated the responses in the roots. In addition, endogenous JA acted as a positive regulator for the transportation of sodium ions from the roots to the shoots because the mutant opr7opr8 had a higher level of sodium in the roots but a significantly lower level in the shoots than WT. Furthermore, JA may act as a positive regulator for ABA biosynthesis in the leaves under salt stress.
Keywords: ABA biosynthesis; ROS; Zea mays; jasmonate; proline; salt response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wani S.H., Kumar V., Shriram V., Sah S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016;4:162–176. doi: 10.1016/j.cj.2016.01.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

