Transthyretin Anti-Amyloidogenic and Fibril Disrupting Activities of Bacopa monnieri (L.) Wettst (Brahmi) Extract
- PMID: 31835306
- PMCID: PMC6995577
- DOI: 10.3390/biom9120845
Transthyretin Anti-Amyloidogenic and Fibril Disrupting Activities of Bacopa monnieri (L.) Wettst (Brahmi) Extract
Abstract
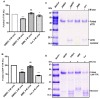
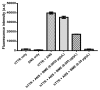
The homotetrameric plasma protein transthyretin (TTR), is responsible for a series of debilitating and often fatal disorders in humans known as transthyretin amyloidosis. Currently, there is no cure for TTR amyloidosis and treatment options are rare. Thus, the identification and development of effective and safe therapeutic agents remain a research imperative. The objective of this study was to determine the effectiveness of Bacopa monnieri extract (BME) in the modulation of TTR amyloidogenesis and disruption of preformed fibrils. Using aggregation assays and transmission electron microscopy, it was found that BME abrogated the formation of human TTR aggregates and mature fibrils but did not dis-aggregate pre-formed fibrils. Through acid-mediated and urea-mediated denaturation assays, it was revealed that BME mitigated the dissociation of folded human TTR and L55P TTR into monomers. ANS binding and glutaraldehyde cross-linking assays showed that BME binds at the thyroxine-binding site and possibly enhanced the quaternary structural stability of native TTR. Together, our results suggest that BME bioactives prevented the formation of TTR fibrils by attenuating the disassembly of tetramers into monomers. These findings open up the possibility of further exploration of BME as a potential resource of valuable anti-TTR amyloidosis therapeutic ingredients.
Keywords: Bacopa monnieri; amyloidosis; antioxidants; bioactive compounds; degenerative diseases; fibrillation; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Trapping the monomer of a non-amyloidogenic variant of transthyretin: exploring its possible use as a therapeutic strategy against transthyretin amyloidogenic diseases.J Biol Chem. 2009 Jan 16;284(3):1443-53. doi: 10.1074/jbc.M807100200. Epub 2008 Nov 4. J Biol Chem. 2009. PMID: 18984591
-
Characterization of the transthyretin acid denaturation pathways by analytical ultracentrifugation: implications for wild-type, V30M, and L55P amyloid fibril formation.Biochemistry. 1998 Dec 22;37(51):17851-64. doi: 10.1021/bi981876+. Biochemistry. 1998. PMID: 9922152
-
Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: the relationship between stability and amyloidosis.Biochemistry. 2008 Jul 1;47(26):6969-84. doi: 10.1021/bi800636q. Epub 2008 Jun 7. Biochemistry. 2008. PMID: 18537267 Free PMC article.
-
Transthyretin Misfolding, A Fatal Structural Pathogenesis Mechanism.Int J Mol Sci. 2021 Apr 23;22(9):4429. doi: 10.3390/ijms22094429. Int J Mol Sci. 2021. PMID: 33922648 Free PMC article. Review.
-
Transthyretin mutagenesis: impact on amyloidogenesis and disease.Crit Rev Clin Lab Sci. 2024 Nov;61(7):616-640. doi: 10.1080/10408363.2024.2350379. Epub 2024 Jun 7. Crit Rev Clin Lab Sci. 2024. PMID: 38850014 Review.
Cited by
-
Peppermint extract inhibits protein aggregation.Biol Futur. 2021 Sep;72(3):367-372. doi: 10.1007/s42977-021-00086-0. Epub 2021 May 4. Biol Futur. 2021. PMID: 34554557
-
Neuroprotective effect against amyloidogenic transthyretin aggregates - Induced cytotoxicity on human neuroblastoma cell by phenolic-rich Centella asiatica extract.Heliyon. 2024 Oct 9;10(20):e39159. doi: 10.1016/j.heliyon.2024.e39159. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640739 Free PMC article.
-
Upcycling of Defatted Sesame Seed Meal via Protein Amyloid-Based Nanostructures: Preparation, Characterization, and Functional and Antioxidant Attributes.Foods. 2024 Jul 20;13(14):2281. doi: 10.3390/foods13142281. Foods. 2024. PMID: 39063365 Free PMC article.
-
Antiproliferative Activities of the Lipophilic Fraction of Eucalyptus camaldulensis against MCF-7 Breast Cancer Cells, UPLC-ESI-QTOF-MS Metabolite Profile, and Antioxidative Functions.ACS Omega. 2022 Jul 29;7(31):27369-27381. doi: 10.1021/acsomega.2c02389. eCollection 2022 Aug 9. ACS Omega. 2022. PMID: 35967023 Free PMC article.
References
-
- Sant’Anna R., Almeida M.R., Varejāo N., Gallego P., Esperante S., Ferreira P., Pereira-Henriques A., Palhano F.L., de Carvalho M., Foguel D., et al. Cavity filling mutations at the thyroxine-binding site dramatically increase transthyretin stability and prevent its aggregation. Sci. Rep. 2017;7:44709. doi: 10.1038/srep44709. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

