Chasing ChEs-MAO B Multi-Targeting 4-Aminomethyl-7-Benzyloxy-2 H-Chromen-2-ones
- PMID: 31835376
- PMCID: PMC6943664
- DOI: 10.3390/molecules24244507
Chasing ChEs-MAO B Multi-Targeting 4-Aminomethyl-7-Benzyloxy-2 H-Chromen-2-ones
Abstract
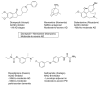
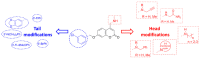
A series of 4-aminomethyl-7-benzyloxy-2H-chromen-2-ones was investigated with the aim of identifying multiple inhibitors of cholinesterases (acetyl- and butyryl-, AChE and BChE) and monoamine oxidase B (MAO B) as potential anti-Alzheimer molecules. Starting from a previously reported potent MAO B inhibitor (3), we studied single-point modifications at the benzyloxy or at the basic moiety. The in vitro screening highlighted triple-acting compounds (6, 8, 9, 16, 20) showing nanomolar and selective MAO B inhibition along with IC50 against ChEs at the low micromolar level. Enzyme kinetics analysis toward AChE and docking simulations on the target enzymes were run in order to get insight into the mechanism of action and plausible binding modes.
Keywords: acetylcholinesterase inhibitors; butyrylcholinesterase inhibitors; coumarin derivatives; docking simulations; monoamine oxidase B inhibitors; multi-target-directed ligands; structure–activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Prince M., Comas-Herrera A., Knapp M., Guerchet M., Karagiannidou M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. Alzheimer’s Disease International (ADI); London, UK: 2016. pp. 1–140.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

