Effect of Myclobutanil Pesticide on the Physiological Behavior of Two Newly Isolated Saccharomyces cerevisiae Strains during Very-High-Gravity Alcoholic Fermentation
- PMID: 31835377
- PMCID: PMC6956295
- DOI: 10.3390/microorganisms7120666
Effect of Myclobutanil Pesticide on the Physiological Behavior of Two Newly Isolated Saccharomyces cerevisiae Strains during Very-High-Gravity Alcoholic Fermentation
Abstract
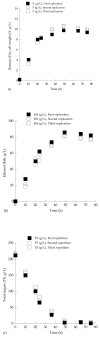
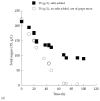
Yeasts are able to act as biosorbents, as their cell wall includes several components capable of binding organic xenobiotic compounds that can potentially be removed during various fermentation processes. In the present investigation, two novel Saccharomyces cerevisiae strains (LMBF-Y 16 and LMBF-Y-18), previously isolated from grapes, were studied regarding their physiological behavior (dry cell weight-DCW production, substrate uptake, and ethanol and glycerol biosynthesis) during fermentations of grape must, in some cases enriched with commercial glucose and fructose (initial total sugar concentration approximately 150 and 250 g/L, respectively). Myclobutanil (a chiral triazole fungicide broadly used as a protective agent of vine) was also added to the culture media at various concentrations in order to assess the ability of the yeasts to simultaneously perform alcoholic fermentations and detoxify the medium (i.e., to remove the fungicide). In the first set of experiments and for both tested strains, trials were carried out in either 250 mL or 2.0 L agitated shake flasks in either synthetic glucose-based experiments or grape musts. Since the results obtained in the trials where the cultures were placed in 2.0 L flasks with grape musts as substrates were superior in terms of both DCW and ethanol production, these experimental conditions were selected for the subsequent studies. Both strains showed high fermentative efficiency, producing high amounts of DCW (9.5-10.5 g/L) in parallel with high ethanol production, which in some cases achieved values very close to the maximum theoretical ethanol production yield (≈0.49 g of ethanol per g of sugar). When using grape must with initial total sugars at approximately 250 g/L (very high gravity fermentation media, close to winemaking conditions), significantly high ethanol quantities (i.e., ranging between 105 and 123 g/L) were produced. Myclobutanil addition slightly negatively affected sugar conversion into ethanol; however, in all cases, ethanol production was very satisfactory. A non-negligible myclobutanil removal during fermentation, which ranged between 5%-27%, as a result of the adsorptive or degradative capacity of the yeast was also reported. The presence of myclobutanil had no effect on DCW production and resulted in no significant differences in the biosynthesis of glycerol. Therefore, these newly isolated yeast strains could be excellent candidates for simultaneous high ethanol production and parallel pesticide removal in a general biorefinery concept demonstrating many environmental benefits.
Keywords: Saccharomyces cerevisiae; ethanol production; myclobutanil; very-high-gravity fermentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains.Microorganisms. 2023 Sep 6;11(9):2243. doi: 10.3390/microorganisms11092243. Microorganisms. 2023. PMID: 37764087 Free PMC article.
-
Growth and metabolism of non-Saccharomyces yeasts isolated from Washington state vineyards in media and high sugar grape musts.Food Microbiol. 2019 Feb;77:158-165. doi: 10.1016/j.fm.2018.09.004. Epub 2018 Sep 4. Food Microbiol. 2019. PMID: 30297046
-
Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations.Int J Food Microbiol. 2019 Sep 16;305:108255. doi: 10.1016/j.ijfoodmicro.2019.108255. Epub 2019 Jun 20. Int J Food Microbiol. 2019. PMID: 31252247
-
Ethanol tolerance in yeasts.Crit Rev Microbiol. 1986;13(3):219-80. doi: 10.3109/10408418609108739. Crit Rev Microbiol. 1986. PMID: 3533426 Review.
-
Yeast's balancing act between ethanol and glycerol production in low-alcohol wines.Microb Biotechnol. 2017 Mar;10(2):264-278. doi: 10.1111/1751-7915.12488. Epub 2017 Jan 13. Microb Biotechnol. 2017. PMID: 28083938 Free PMC article. Review.
Cited by
-
Cell wall modifications in Saccharomyces cerevisiae wine yeast through adaptive laboratory evolution with Tebuconazole.Sci Rep. 2025 Jul 15;15(1):25438. doi: 10.1038/s41598-025-11080-0. Sci Rep. 2025. PMID: 40659902 Free PMC article.
-
Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review.Molecules. 2020 Aug 13;25(16):3698. doi: 10.3390/molecules25163698. Molecules. 2020. PMID: 32823772 Free PMC article. Review.
-
Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality.Microorganisms. 2020 May 20;8(5):764. doi: 10.3390/microorganisms8050764. Microorganisms. 2020. PMID: 32443782 Free PMC article.
References
-
- Camarasa C., Chiron H., Daboussi F., Della Valle G., Dumas C., Farines V., Floury J., Gagnaire V., Gorret N., Leonil J., et al. INRA’s research in industrial biotechnology: For food, chemicals, materials and fuels. Innov. Food Sci. Emerg. Technol. 2018;46:140–152. doi: 10.1016/j.ifset.2017.11.008. - DOI
-
- Koutinas A.A., Wang R.H., Webb C. The biochemurgist -Bioconversion of agricultural raw materials for chemical production. Biofuels Bioprod. Biorefining. 2007;1:24–38. doi: 10.1002/bbb.6. - DOI
-
- Sarris D., Papanikolaou S. Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016;16:307–329. doi: 10.1002/elsc.201400199. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

