Alcohol Metabolism Potentiates HIV-Induced Hepatotoxicity: Contribution to End-Stage Liver Disease
- PMID: 31835520
- PMCID: PMC6995634
- DOI: 10.3390/biom9120851
Alcohol Metabolism Potentiates HIV-Induced Hepatotoxicity: Contribution to End-Stage Liver Disease
Abstract
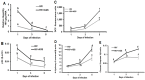
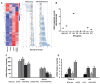
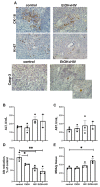
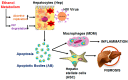
In an era of improved survival due to modern antiretroviral therapy, liver disease has become a major cause of morbidity and mortality, resulting in death in 15-17% of human immunodeficiency virus (HIV)-infected patients. Alcohol enhances HIV-mediated liver damage and promotes the progression to advanced fibrosis and cirrhosis. However, the mechanisms behind these events are uncertain. Here, we hypothesize that ethanol metabolism potentiates accumulation of HIV in hepatocytes, causing oxidative stress and intensive apoptotic cell death. Engulfment of HIV-containing apoptotic hepatocytes by non-parenchymal cells (NPCs) triggers their activation and liver injury progression. This study was performed on primary human hepatocytes and Huh7.5-CYP cells infected with HIV-1ADA, and major findings were confirmed by pilot data obtained on ethanol-fed HIV-injected chimeric mice with humanized livers. We demonstrated that ethanol exposure potentiates HIV accumulation in hepatocytes by suppressing HIV degradation by lysosomes and proteasomes. This leads to increased oxidative stress and hepatocyte apoptosis. Exposure of HIV-infected apoptotic hepatocytes to NPCs activates the inflammasome in macrophages and pro-fibrotic genes in hepatic stellate cells. We conclude that while HIV and ethanol metabolism-triggered apoptosis clears up HIV-infected hepatocytes, continued generation of HIV-expressing apoptotic bodies may be detrimental for progression of liver inflammation and fibrosis due to constant activation of NPCs.
Keywords: acetaldehyde; apoptotic bodies; ethanol; fibrosis; hepatocytes; human immunodeficiency virus; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


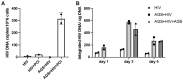



References
-
- Pascual-Pareja J.F., Caminoa A., Larrauri C., Gonzalez-Garcia J., Montes M.L., Diez J., Grande M., Arribas J.R. Haart is associated with lower hepatic necroinflammatory activity in hiv-hepatitis c virus-coinfected patients with cd4 cell count of more than 350 cells/microl at the time of liver biopsy. AIDS. 2009;23:971–975. doi: 10.1097/QAD.0b013e328329f994. - DOI - PubMed
-
- Mata-Marin J.A., Gaytan-Martinez J., Grados-Chavarria B.H., Fuentes-Allen J.L., Arroyo-Anduiza C.I., Alfaro-Mejia A. Correlation between hiv viral load and aminotransferases as liver damage markers in hiv infected naive patients: A concordance cross-sectional study. Virol. J. 2009;6:181. doi: 10.1186/1743-422X-6-181. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

