Maternal Yes-Associated Protein Participates in Porcine Blastocyst Development via Modulation of Trophectoderm Epithelium Barrier Function
- PMID: 31835702
- PMCID: PMC6952962
- DOI: 10.3390/cells8121606
Maternal Yes-Associated Protein Participates in Porcine Blastocyst Development via Modulation of Trophectoderm Epithelium Barrier Function
Abstract
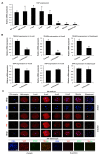
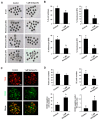
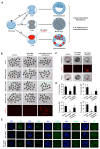
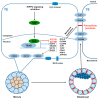
The establishment of a functional trophectoderm (TE) epithelium is an essential prerequisite for blastocyst formation and placentation. Transcription coactivator yes-associated protein (YAP), a downstream effector of the hippo signaling pathway, is required for specification of both the TE and epiblast lineages in mice. However, the biological role of YAP in porcine blastocyst development is not known. Here, we report that maternally derived YAP protein is localized to both the cytoplasm and nuclei prior to the morula stage and is then predominantly localized to the TE nuclei in blastocysts. Functionally, maternal YAP knockdown severely impeded blastocyst formation and perturbed the allocation of the first two lineages. The treatment of embryos with verteporfin, a pharmacological inhibitor of YAP, faithfully recapitulated the phenotype observed in YAP deleted embryos. Mechanistically, we found that maternal YAP regulates multiple genes which are important for lineage commitment, tight junction assembly, and fluid accumulation. Consistent with the effects on tight junction gene expression, a permeability assay revealed that paracellular sealing was defective in the trophectoderm epithelium. Lastly, YAP knockdown in a single blastomere at the 2-cell stage revealed that the cellular progeny of the YAP+ blastomere were sufficient to sustain blastocyst formation via direct complementation of the defective trophectoderm epithelium. In summary, these findings demonstrate that maternal YAP facilitates porcine blastocyst development through transcriptional regulation of key genes that are essential for lineage commitment, tight junction assembly, and fluid accumulation.
Keywords: YAP; blastocyst development; pig; tight junction; trophectoderm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

