Defining decreased protein succinylation of failing human cardiac myofibrils in ischemic cardiomyopathy
- PMID: 31836543
- PMCID: PMC7058372
- DOI: 10.1016/j.yjmcc.2019.11.159
Defining decreased protein succinylation of failing human cardiac myofibrils in ischemic cardiomyopathy
Abstract
Succinylation is a post-translational modification of protein lysine residues with succinyl groups derived from succinyl CoA. Succinylation is considered a significant post-translational modification with the potential to impact protein function which is highly conserved across numerous species. The role of succinylation in the heart, especially in heart failure and myofibril mechanics, remains largely unexplored. Mechanical parameters were measured in myofibrils isolated from failing hearts of ischemic cardiomyopathy patients and non-failing donor controls. We employed mass spectrometry to quantify differential protein expression in myofibrils from failing ischemic cardiomyopathy hearts compared to non-failing hearts. In addition, we combined peptide enrichment by immunoprecipitation with liquid chromatography tandem mass spectrometry to quantitatively analyze succinylated lysine residues in these myofibrils. Several key parameters of sarcomeric mechanical interactions were altered in myofibrils isolated from failing ischemic cardiomyopathy hearts, including lower resting tension and a faster rate of activation. Of the 100 differentially expressed proteins, 46 showed increased expression in ischemic heart failure, while 54 demonstrated decreased expression in ischemic heart failure. Our quantitative succinylome analysis identified a total of 572 unique succinylated lysine sites located on 181 proteins, with 307 significantly changed succinylation events. We found that 297 succinyl-Lys demonstrated decreased succinylation on 104 proteins, while 10 residues demonstrated increased succinylation on 4 proteins. Investigating succinyl CoA generation, enzyme activity assays demonstrated that α-ketoglutarate dehydrogenase and succinate dehydrogenase activities were significantly decreased in ischemic heart failure. An activity assay for succinyl CoA synthetase demonstrated a significant increase in ischemic heart failure. Taken together, our findings support the hypothesis that succinyl CoA production is decreased and succinyl CoA turnover is increased in ischemic heart failure, potentially resulting in an overall decrease in the mitochondrial succinyl CoA pool, which may contribute to decreased myofibril protein succinylation in heart failure.
Keywords: Heart failure; Myofibrils; Post-translational modification; Proteomics; Sirtuins; Succinylation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflict of interest to declare.
Figures
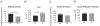





References
-
- Klabunde RE. Cardiovascular physiology concepts. 2nd ed Philadelphis, PA: Lippincott Williams &Wilkins/Wolters Kluwer; 2011. 2011.
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131(4):e29–e322. - PubMed
-
- Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. European journal of heart failure. 2017;19(9):1095–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

