Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application
- PMID: 31836580
- PMCID: PMC7028964
- DOI: 10.1128/AEM.02749-19
Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application
Abstract
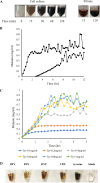
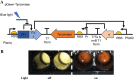
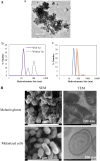
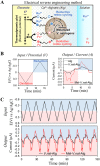
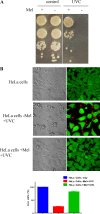
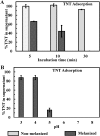
Melanin is a pigment produced by organisms throughout all domains of life. Due to its unique physicochemical properties, biocompatibility, and biostability, there has been an increasing interest in the use of melanin for broad applications. In the vast majority of studies, melanin has been either chemically synthesized or isolated from animals, which has restricted its use to small-scale applications. Using bacteria as biocatalysts is a promising and economical alternative for the large-scale production of biomaterials. In this study, we engineered the marine bacterium Vibrio natriegens, one of the fastest-growing organisms, to synthesize melanin by expressing a heterologous tyrosinase gene and demonstrated that melanin production was much faster than in previously reported heterologous systems. The melanin of V. natriegens was characterized as a polymer derived from dihydroxyindole-2-carboxylic acid (DHICA) and, similarly to synthetic melanin, exhibited several characteristic and useful features. Electron microscopy analysis demonstrated that melanin produced from V. natriegens formed nanoparticles that were assembled as "melanin ghost" structures, and the photoprotective properties of these particles were validated by their protection of cells from UV irradiation. Using a novel electrochemical reverse engineering method, we observed that melanization conferred redox activity to V. natriegens Moreover, melanized bacteria were able to quickly adsorb the organic compound trinitrotoluene (TNT). Overall, the genetic tractability, rapid division time, and ease of culture provide a set of attractive properties that compare favorably to current E. coli production strains and warrant the further development of this chassis as a microbial factory for natural product biosynthesis.IMPORTANCE Melanins are macromolecules that are ubiquitous in nature and impart a large variety of biological functions, including structure, coloration, radiation resistance, free radical scavenging, and thermoregulation. Currently, in the majority of investigations, melanins are either chemically synthesized or extracted from animals, which presents significant challenges for large-scale production. Bacteria have been used as biocatalysts to synthesize a variety of biomaterials due to their fast growth and amenability to genetic engineering using synthetic biology tools. In this study, we engineered the extremely fast-growing bacterium V. natriegens to synthesize melanin nanoparticles by expressing a heterologous tyrosinase gene with inducible promoters. Characterization of the melanin produced from V. natriegens-produced tyrosinase revealed that it exhibited physical and chemical properties similar to those of natural and chemically synthesized melanins, including nanoparticle structure, protection against UV damage, and adsorption of toxic compounds. We anticipate that producing and controlling melanin structures at the nanoscale in this bacterial system with synthetic biology tools will enable the design and rapid production of novel biomaterials for multiple applications.
Keywords: Vibrio natriegens; biomanufacturing; fast growing; melanin; melanin biosynthesis; nanoparticle; synthetic biology.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- d'Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, Galván I, Ghanem G, Kenzo K, Meredith P, Pezzella A, Santato C, Sarna T, Simon JD, Zecca L, Zucca FA, Napolitano A, Ito S. 2015. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res 28:520–544. doi:10.1111/pcmr.12393. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

