Identification of the Genetic Requirements for Zinc Tolerance and Toxicity in Saccharomyces cerevisiae
- PMID: 31836620
- PMCID: PMC7003084
- DOI: 10.1534/g3.119.400933
Identification of the Genetic Requirements for Zinc Tolerance and Toxicity in Saccharomyces cerevisiae
Abstract
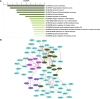
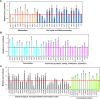
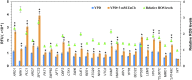
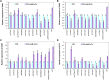
Zinc is essential for almost all living organisms, since it serves as a crucial cofactor for transcription factors and enzymes. However, it is toxic to cell growth when present in excess. The present work aims to investigate the toxicity mechanisms induced by zinc stress in yeast cells. To this end, 108 yeast single-gene deletion mutants were identified sensitive to 6 mM ZnCl2 through a genome-wide screen. These genes were predominantly related to the biological processes of vacuolar acidification and transport, polyphosphate metabolic process, cytosolic transport, the process utilizing autophagic mechanism. A result from the measurement of intracellular zinc content showed that 64 mutants accumulated higher intracellular zinc under zinc stress than the wild-type cells. We further measured the intracellular ROS (reactive oxygen species) levels of 108 zinc-sensitive mutants treated with 3 mM ZnCl2 We showed that the intracellular ROS levels in 51 mutants were increased by high zinc stress, suggesting their possible involvement in regulating ROS homeostasis in response to high zinc. The results also revealed that excess zinc could generate oxidative damage and then activate the expression of several antioxidant defenses genes. Taken together, the data obtained indicated that excess zinc toxicity might be mainly due to the high intracellular zinc levels and ROS levels induced by zinc stress in yeast cells. Our current findings would provide a basis to understand the molecular mechanisms of zinc toxicity in yeast cells.
Keywords: Reactive oxygen species (ROS); Saccharomyces cerevisiae; genetic screening; genomics; zinc toxicity.
Copyright © 2020 Zhao et al.
Figures
Similar articles
-
Disruption of iron homeostasis in Saccharomyces cerevisiae by high zinc levels: a genome-wide study.Mol Microbiol. 2007 Jul;65(2):521-37. doi: 10.1111/j.1365-2958.2007.05807.x. Mol Microbiol. 2007. PMID: 17630978
-
Genome-wide identification for genes involved in sodium dodecyl sulfate toxicity in Saccharomyces cerevisiae.BMC Microbiol. 2020 Feb 17;20(1):34. doi: 10.1186/s12866-020-1721-2. BMC Microbiol. 2020. PMID: 32066383 Free PMC article.
-
Vacuolar H+-ATPase Protects Saccharomyces cerevisiae Cells against Ethanol-Induced Oxidative and Cell Wall Stresses.Appl Environ Microbiol. 2016 May 2;82(10):3121-3130. doi: 10.1128/AEM.00376-16. Print 2016 May 15. Appl Environ Microbiol. 2016. PMID: 26994074 Free PMC article.
-
Multiple regulatory mechanisms maintain zinc homeostasis in Saccharomyces cerevisiae.J Nutr. 2003 May;133(5 Suppl 1):1532S-5S. doi: 10.1093/jn/133.5.1532S. J Nutr. 2003. PMID: 12730459 Review.
-
Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae.J Biol Chem. 2009 Jul 10;284(28):18565-9. doi: 10.1074/jbc.R900014200. Epub 2009 Apr 10. J Biol Chem. 2009. PMID: 19363031 Free PMC article. Review.
Cited by
-
Metabolomics of bacterial-fungal pairwise interactions reveal conserved molecular mechanisms.Analyst. 2023 Jun 26;148(13):3002-3018. doi: 10.1039/d3an00408b. Analyst. 2023. PMID: 37259951 Free PMC article.
-
Expression of the H2O2 Biosensor roGFP-Tpx1.C160S in Fission and Budding Yeasts and Jurkat Cells to Compare Intracellular H2O2 Levels, Transmembrane Gradients, and Response to Metals.Antioxidants (Basel). 2023 Mar 13;12(3):706. doi: 10.3390/antiox12030706. Antioxidants (Basel). 2023. PMID: 36978953 Free PMC article.
-
FgVAC1 is an Essential Gene Required for Golgi-to-Vacuole Transport and Fungal Development in Fusarium graminearum.J Microbiol. 2024 Aug;62(8):649-660. doi: 10.1007/s12275-024-00160-x. Epub 2024 Jul 30. J Microbiol. 2024. PMID: 39080148 Free PMC article.
-
A Systematic Survey of Characteristic Features of Yeast Cell Death Triggered by External Factors.J Fungi (Basel). 2021 Oct 20;7(11):886. doi: 10.3390/jof7110886. J Fungi (Basel). 2021. PMID: 34829175 Free PMC article. Review.
-
Multi trace element profiling in pathogenic and non-pathogenic fungi.Fungal Biol. 2020 May;124(5):516-524. doi: 10.1016/j.funbio.2020.03.001. Epub 2020 Mar 10. Fungal Biol. 2020. PMID: 32389315 Free PMC article.
References
-
- Bun-ya M., Shikata K., Nakade S., Yompakdee C., Harashima S. et al. , 1996. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr. Genet. 29: 344–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
