Gene therapy for progressive familial intrahepatic cholestasis type 3 in a clinically relevant mouse model
- PMID: 31836711
- PMCID: PMC6910969
- DOI: 10.1038/s41467-019-13614-3
Gene therapy for progressive familial intrahepatic cholestasis type 3 in a clinically relevant mouse model
Abstract
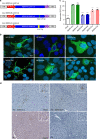
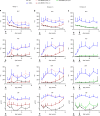
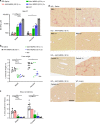
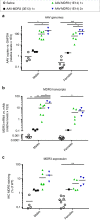
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is a rare monogenic disease caused by mutations in the ABCB4 gene, resulting in a reduction in biliary phosphatidylcholine. Reduced biliary phosphatidylcholine cannot counteract the detergent effects of bile salts, leading to cholestasis, cholangitis, cirrhosis and ultimately liver failure. Here, we report results from treating two- or five-week-old Abcb4-/- mice with an AAV vector expressing human ABCB4, resulting in significant decreases of PFIC3 disease biomarkers. All male mice achieved a sustained therapeutic effect up through 12 weeks, but the effect was achieved in only 50% of females. However, two-week-old females receiving a second inoculation three weeks later maintained the therapeutic effect. Upon sacrifice, markers of PFIC3 disease such as, hepatosplenomegaly, biliary phosphatidylcholine and liver histology were significantly improved. Thus, AAV-mediated gene therapy successfully prevented PFIC3 symptoms in a clinically relevant mouse model, representing a step forward in improving potential therapy options for PFIC3 patients.
Conflict of interest statement
Drs. Weber, Ferrer, Douar, Bénichou, and González-Aseguinolaza are all employees of Vivet Therapeutics, a startup biopharmaceutical company developing gene therapy approaches for treatment of rare metabolic diseases. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

