E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes
- PMID: 31836726
- PMCID: PMC6910911
- DOI: 10.1038/s41598-019-51643-6
E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes
Abstract
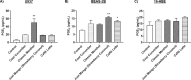
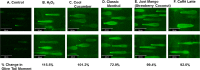
E-cigarette flavored pods are increasing in use among young adults. Although marketed as a safer alternative to conventional cigarettes, the health effects of e-cigarette flavored pods are unknown. We hypothesized that e-cigarette flavored pods would cause oxidative stress, barrier dysfunction, and an inflammatory response in monocytes and lung epithelial cells. JUUL pod flavors (Fruit Medley, Virginia Tobacco, Cool Mint, Crème Brulee, Cool Cucumber, Mango, and Classic Menthol) and similar pod flavors (Just Mango-Strawberry Coconut and Caffé Latte) were tested. These pod flavors generated significant amounts of acellular ROS and induced significant mitochondrial superoxide production in bronchial epithelial cells (16-HBE). Lung epithelial cells (16-HBE, BEAS-2B) and monocytes (U937) exposed to various pod aerosols resulted in increased inflammatory mediators, such as IL-8 or PGE2. JUUL pod flavors, Crème Brulee and Cool Cucumber, caused epithelial barrier dysfunction in 16-HBE cells. Moreover, tested flavors also showed DNA damage upon exposure in monocytes. We determined the chemical constituents present in various flavors. Our data suggest that these constituents in flavored pods induce oxidative stress, inflammation, epithelial barrier dysfunction, and DNA damage in lung cells. These data provide insights into the regulation of e-cigarette flavored pods, as well as constituents in these flavors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bals Robert, Boyd Jeanette, Esposito Susanna, Foronjy Robert, Hiemstra Pieter S., Jiménez-Ruiz Carlos A., Katsaounou Paraskevi, Lindberg Anne, Metz Carlos, Schober Wolfgang, Spira Avrum, Blasi Francesco. Electronic cigarettes: a task force report from the European Respiratory Society. European Respiratory Journal. 2019;53(2):1801151. doi: 10.1183/13993003.01151-2018. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 ES007026/ES/NIEHS NIH HHS/United States
- U54CA228110/National Cancer Center/International
- 1R01HL135613/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)/International
- T32-ES00702/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)/International
LinkOut - more resources
Full Text Sources
Medical

