Selective synthesis of spirobiindanes, alkenyl chlorides, and monofluoroalkenes from unactivated gem-difluoroalkanes controlled by aluminum-based Lewis acids
- PMID: 31836738
- PMCID: PMC6911048
- DOI: 10.1038/s41598-019-55206-7
Selective synthesis of spirobiindanes, alkenyl chlorides, and monofluoroalkenes from unactivated gem-difluoroalkanes controlled by aluminum-based Lewis acids
Abstract
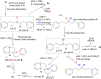
The highly selective synthesis of spirobiindanes, alkenyl chlorides, and monofluoroalkenes via the cleavage of inert C(sp3)-F bonds in unactivated gem-difluoroalkanes using readily available and inexpensive aluminum-based Lewis acids of low toxicity is reported. The selectivity of this reaction can be controlled by modifying the substituents on the central aluminum atom of the promoter. An intramolecular cascade Friedel-Crafts alkylation of unactivated gem-difluorocarbons can be achieved using a stoichiometric amount of AlCl3. The subsequent synthesis of alkenyl chlorides via F/Cl exchange followed by an elimination can be accomplished using AlEt2Cl as a fluoride scavenger and halogen source. The defluorinative elimination of acyclic and cyclic gem-difluorocarbons to give monofluoroalkenes can be achieved using AlEt3.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Activation of Saturated Fluorocarbons to Synthesize Spirobiindanes, Monofluoroalkenes, and Indane Derivatives.iScience. 2019 Jul 26;17:132-143. doi: 10.1016/j.isci.2019.06.018. Epub 2019 Jun 19. iScience. 2019. PMID: 31276957 Free PMC article.
-
Synthesis of Alkylated Monofluoroalkenes via Fe-Catalyzed Defluorinative Cross-Coupling of Donor Alkenes with gem-Difluoroalkenes.Org Lett. 2018 Apr 6;20(7):1924-1927. doi: 10.1021/acs.orglett.8b00471. Epub 2018 Mar 19. Org Lett. 2018. PMID: 29552884
-
Nickel-Catalyzed Defluorinative Reductive Cross-Coupling of gem-Difluoroalkenes with Unactivated Secondary and Tertiary Alkyl Halides.J Am Chem Soc. 2017 Sep 13;139(36):12632-12637. doi: 10.1021/jacs.7b06469. Epub 2017 Aug 29. J Am Chem Soc. 2017. PMID: 28849923
-
Transition metal-catalyzed C(sp2/sp3)-H α-fluoroalkenylation from gem-(bromo/di)fluoroalkenes to monofluoroalkenes: scope, mechanisms, and synthetic applications.Org Biomol Chem. 2024 Aug 28;22(34):6860-6904. doi: 10.1039/d4ob01044b. Org Biomol Chem. 2024. PMID: 39136141 Review.
-
Iodine(III)-Mediated Migratory gem-Difluorinations: Synthesis of β Transformable Functionality Substituted gem-Difluoroalkanes.Chem Rec. 2023 Dec;23(12):e202300231. doi: 10.1002/tcr.202300231. Epub 2023 Sep 4. Chem Rec. 2023. PMID: 37665225 Review.
Cited by
-
Catalyst-free carbosilylation of alkenes using silyl boronates and organic fluorides via selective C-F bond activation.Nat Commun. 2021 Jun 18;12(1):3749. doi: 10.1038/s41467-021-24031-w. Nat Commun. 2021. PMID: 34145264 Free PMC article.
-
Unexpected rearrangements and a novel synthesis of 1,1-dichloro-1-alkenones from 1,1,1-trifluoroalkanones with aluminium trichloride.Beilstein J Org Chem. 2021 Feb 10;17:404-409. doi: 10.3762/bjoc.17.36. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33633808 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

