Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1-42), pTau and tTau CSF immunoassays
- PMID: 31836810
- PMCID: PMC6911086
- DOI: 10.1038/s41598-019-54204-z
Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1-42), pTau and tTau CSF immunoassays
Abstract
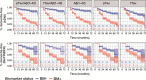
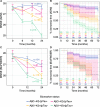
We evaluated the performance of CSF biomarkers for predicting risk of clinical decline and conversion to dementia in non-demented patients with cognitive symptoms. CSF samples from patients in two multicentre longitudinal studies (ADNI, n = 619; BioFINDER, n = 431) were analysed. Aβ(1-42), tTau and pTau CSF concentrations were measured using Elecsys CSF immunoassays, and tTau/Aβ(1-42) and pTau/Aβ(1-42) ratios calculated. Patients were classified as biomarker (BM)-positive or BM-negative at baseline. Ability of biomarkers to predict risk of clinical decline and conversion to AD/dementia was assessed using pre-established cut-offs for Aβ(1-42) and ratios; tTau and pTau cut-offs were determined. BM-positive patients showed greater clinical decline than BM-negative patients, demonstrated by greater decreases in MMSE scores (all biomarkers: -2.10 to -0.70). Risk of conversion to AD/dementia was higher in BM-positive patients (HR: 1.67 to 11.48). Performance of Tau/Aβ(1-42) ratios was superior to single biomarkers, and consistent even when using cut-offs derived in a different cohort. Optimal pTau and tTau cut-offs were approximately 27 pg/mL and 300 pg/mL in both BioFINDER and ADNI. Elecsys pTau/Aβ(1-42) and tTau/Aβ(1-42) are robust biomarkers for predicting risk of clinical decline and conversion to dementia in non-demented patients, and may support AD diagnosis in clinical practice.
Conflict of interest statement
K.B. served as a consultant or at advisory boards for Alzheon, BioArctic, Roche Diagnostics, Eli Lilly, Fujirebio Europe, Merck, Novartis and IBL International. His research team has received funds for research from Roche Diagnostics. He is the co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. L.M.S. received research support from NIH/NIA, ADNI (AG024904) and UPenn ADCC Biomarker Core (AG010124), MJFox Foundation for PD research, Roche, Lilly; provides QC oversight for Roche Elecsys CSF AD biomarker immunoassays for ADNI; and is a consultant for Roche, Lilly, Novartis. E.S., N.M., J.B.T. and J.Q.T. declare that they have no conflict of interest. S.W., U.E., V.L., M.S., and K.Bu. are Roche employees. O.H. acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, Fujirebio and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, Roche and Fujirebio.
Figures

References
-
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
-
- Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

