Sotagliflozin Decreases Postprandial Glucose and Insulin Concentrations by Delaying Intestinal Glucose Absorption
- PMID: 31837264
- PMCID: PMC7067537
- DOI: 10.1210/clinem/dgz258
Sotagliflozin Decreases Postprandial Glucose and Insulin Concentrations by Delaying Intestinal Glucose Absorption
Abstract
Context: The effect of sotagliflozin (a dual sodium-glucose cotransporter [SGLT] 2 and SGLT1 inhibitor) on intestinal glucose absorption has not been investigated in humans.
Objective: To measure rate of appearance of oral glucose (RaO) using a dual glucose tracer method following standardized mixed meals taken after single sotagliflozin or canagliflozin doses.
Setting: Clinical research organization.
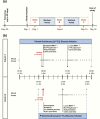
Design and participants: In a double-blind, 3-period crossover study (NCT01916863), 24 healthy participants were randomized to 2 cohorts of 12 participants. Within each cohort, participants were randomly assigned single oral doses of either sotagliflozin 400 mg, canagliflozin 300 mg, or placebo on each of test days 1, 8, and 15. On test days, Cohort 1 had breakfast containing [6,6-2H2] glucose 0.25 hours postdose and lunch containing [1-2H1] glucose 5.25 hours postdose; Cohort 2 had breakfast containing no labeled glucose 0.25 hours postdose and lunch containing [6,6-2H2] glucose 4.25 hours postdose. All participants received a 10- to 15-hour continuous [U-13C6] glucose infusion starting 5 hours before their first [6,6-2H2] glucose-containing meal.
Main outcome: RaO, postprandial glucose (PPG), and postprandial insulin.
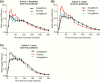
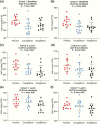
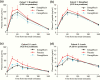
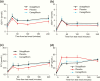
Results: Sotagliflozin and canagliflozin decreased area under the curve (AUC)0-1 hour and/or AUC0-2 hours for RaO, PPG, and insulin after breakfast and/or the 4.25-hour postdose lunch (P < .05 versus placebo). After the 5.25-hour postdose lunch, sotagliflozin lowered RaO AUC0-1 hour and PPG AUC0-5 hours versus both placebo and canagliflozin (P < .05).
Conclusions: Sotagliflozin delayed and blunted intestinal glucose absorption after meals, resulting in lower PPG and insulin levels, likely due to prolonged local inhibition of intestinal SGLT1 that persisted for ≥5 hours after dosing.
Keywords: SGLT1 inhibitor; SGLT2 inhibitor; Sotagliflozin; intestinal glucose absorption; postprandial glucose; type 2 diabetes.
© Endocrine Society 2019.
Figures


References
-
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. - PubMed
-
- Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015;38(12):2344–2353. - PubMed
-
- Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44(4):375–393. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. - PubMed

