A Discrete Presynaptic Vesicle Cycle for Neuromodulator Receptors
- PMID: 31837915
- PMCID: PMC7035187
- DOI: 10.1016/j.neuron.2019.11.016
A Discrete Presynaptic Vesicle Cycle for Neuromodulator Receptors
Abstract
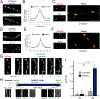
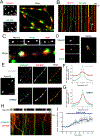
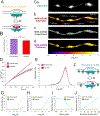
A major function of GPCRs is to inhibit presynaptic neurotransmitter release, requiring ligand-activated receptors to couple locally to effectors at terminals. The current understanding of how this is achieved is through receptor immobilization on the terminal surface. Here, we show that opioid peptide receptors, GPCRs that mediate highly sensitive presynaptic inhibition, are instead dynamic in axons. Opioid receptors diffuse rapidly throughout the axon surface and internalize after ligand-induced activation specifically at presynaptic terminals. We delineate a parallel regulated endocytic cycle for GPCRs operating at the presynapse, separately from the synaptic vesicle cycle, which clears activated receptors from the surface of terminals and locally reinserts them to maintain the diffusible surface pool. We propose an alternate strategy for achieving local control of presynaptic effectors that, opposite to using receptor immobilization and enforced proximity, is based on lateral mobility of receptors and leverages the inherent allostery of GPCR-effector coupling.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, and Grandy DK (2000). Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol. Pharmacol 58, 1570–1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

