Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials
- PMID: 31838015
- PMCID: PMC7811790
- DOI: 10.1016/S1470-2045(19)30690-4
Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials
Erratum in
-
Correction to Lancet Oncol 2020; 21: 261-70.Lancet Oncol. 2020 Feb;21(2):e70. doi: 10.1016/S1470-2045(20)30007-3. Lancet Oncol. 2020. PMID: 32007206 No abstract available.
-
Correction to Lancet Oncol 2020; 21: 261-70.Lancet Oncol. 2020 Jul;21(7):e341. doi: 10.1016/S1470-2045(20)30346-6. Lancet Oncol. 2020. PMID: 32615113 No abstract available.
Abstract
Background: Recurrent gene fusions, such as ROS1 fusions, are oncogenic drivers of various cancers, including non-small-cell lung cancer (NSCLC). Up to 36% of patients with ROS1 fusion-positive NSCLC have brain metastases at the diagnosis of advanced disease. Entrectinib is a ROS1 inhibitor that has been designed to effectively penetrate and remain in the CNS. We explored the use of entrectinib in patients with locally advanced or metastatic ROS1 fusion-positive NSCLC.
Methods: We did an integrated analysis of three ongoing phase 1 or 2 trials of entrectinib (ALKA-372-001, STARTRK-1, and STARTRK-2). The efficacy-evaluable population included adult patients (aged ≥18 years) with locally advanced or metastatic ROS1 fusion-positive NSCLC who received entrectinib at a dose of at least 600 mg orally once per day, with at least 12 months' follow-up. All patients had an Eastern Cooperative Oncology Group performance status of 0-2, and previous cancer treatment (except for ROS1 inhibitors) was allowed. The primary endpoints were the proportion of patients with an objective response (complete or partial response according to Response Evaluation Criteria in Solid Tumors version 1.1) and duration of response, and were evaluated by blinded independent central review. The safety-evaluable population for the safety analysis included all patients with ROS1 fusion-positive NSCLC in the three trials who received at least one dose of entrectinib (irrespective of dose or duration of follow-up). These ongoing studies are registered with ClinicalTrials.gov, NCT02097810 (STARTRK-1) and NCT02568267 (STARTRK-2), and EudraCT, 2012-000148-88 (ALKA-372-001).
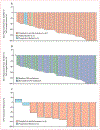
Findings: Patients were enrolled in ALKA-372-001 from Oct 26, 2012, to March 27, 2018; in STARTRK-1 from Aug 7, 2014, to May 10, 2018; and in STARTRK-2 from Nov 19, 2015 (enrolment is ongoing). At the data cutoff date for this analysis (May 31, 2018), 41 (77%; 95% CI 64-88) of 53 patients in the efficacy-evaluable population had an objective response. Median follow-up was 15·5 monhts (IQR 13·4-20·2). Median duration of response was 24·6 months (95% CI 11·4-34·8). In the safety-evaluable population, 79 (59%) of 134 patients had grade 1 or 2 treatment-related adverse events. 46 (34%) of 134 patients had grade 3 or 4 treatment-related adverse events, with the most common being weight increase (ten [8%]) and neutropenia (five [4%]). 15 (11%) patients had serious treatment-related adverse events, the most common of which were nervous system disorders (four [3%]) and cardiac disorders (three [2%]). No treatment-related deaths occurred.
Interpretation: Entrectinib is active with durable disease control in patients with ROS1 fusion-positive NSCLC, and is well tolerated with a manageable safety profile, making it amenable to long-term dosing in these patients. These data highlight the need to routinely test for ROS1 fusions to broaden therapeutic options for patients with ROS1 fusion-positive NSCLC.
Funding: Ignyta/F Hoffmann-La Roche.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Entrectinib for ROS1 fusion-positive NSCLC and NTRK fusion-positive solid tumours.Lancet Oncol. 2020 Feb;21(2):193-194. doi: 10.1016/S1470-2045(19)30789-2. Epub 2019 Dec 11. Lancet Oncol. 2020. PMID: 31838008 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

