Regulation of autophagy by canonical and non-canonical ER stress responses
- PMID: 31838023
- PMCID: PMC7325862
- DOI: 10.1016/j.semcancer.2019.11.007
Regulation of autophagy by canonical and non-canonical ER stress responses
Abstract
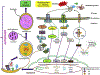
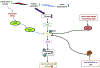
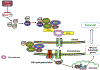
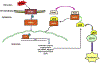
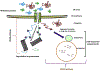
Cancer cells encounter numerous stresses that pose a threat to their survival. Tumor microenviroment stresses that perturb protein homeostasis can produce endoplasmic reticulum (ER) stress, which can be counterbalanced by triggering the unfolded protein response (UPR) which is considered the canonical ER stress response. The UPR is characterized by three major proteins that lead to specific changes in transcriptional and translational programs in stressed cells. Activation of the UPR can induce apoptosis, but also can induce cytoprotective programs such as autophagy. There is increasing appreciation for the role that UPR-induced autophagy plays in supporting tumorigenesis and cancer therapy resistance. More recently several new pathways that connect cell stresses, components of the UPR and autophagy have been reported, which together can be viewed as non-canonical ER stress responses. Here we review recent findings on the molecular mechanisms by which canonical and non-canonical ER stress responses can activate cytoprotective autophagy and contribute to tumor growth and therapy resistance. Autophagy has been identified as a druggable pathway, however the components of autophagy (ATG genes) have proven difficult to drug. It may be the case that targeting the UPR or non-canonical ER stress programs can more effectively block cytoprotective autophagy to enhance cancer therapy. A deeper understanding of these pathways could provide new therapeutic targets in cancer.
Keywords: Autophagy; Cancer; Canonical endoplasmic reticulum stress; Non-canonical endoplasmic reticulum stress; Unfolded protein response.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
References
-
- GM C The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000.
-
- Alberts B, Johnson A, Lewis J ea. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

