Long-Term Comparison Between Pulmonary Homograft Versus Bioprosthesis for Pulmonary Valve Replacement in Tetralogy of Fallot
- PMID: 31838974
- PMCID: PMC6951084
- DOI: 10.1161/JAHA.119.013654
Long-Term Comparison Between Pulmonary Homograft Versus Bioprosthesis for Pulmonary Valve Replacement in Tetralogy of Fallot
Abstract
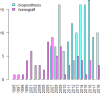
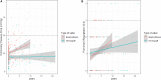
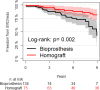
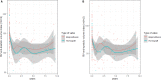
Background Tetralogy of Fallot repair results in late occurrence of pulmonary regurgitation, which requires pulmonary valve replacement in a large proportion of patients. Both homografts and bioprostheses are used for pulmonary valve replacement as uncertainty remains on which prosthesis should be considered superior. We performed a long-term imaging and clinical comparison between these 2 strategies. Methods and Results We compared echocardiographic and clinical follow-up data of 209 patients with previous tetralogy of Fallot repair who underwent pulmonary valve replacement with homograft (n=75) or bioprosthesis (n=134) between 1995 and 2018 at a tertiary hospital. The primary end point was the composite of pulmonary valve replacement reintervention and structural valve deterioration, defined as a transpulmonary pressure decrease ≥50 mm Hg or pulmonary regurgitation degree of ≥2. Mixed linear model and Cox regression model were used for comparisons. Echocardiographic follow-up duration was longer in the homograft group (8 [interquartile range, 4-12] versus 4 [interquartile range, 3-6] years; P<0.001). At the latest echocardiographic follow-up, homografts showed a significantly lower transpulmonary systolic pressure decrease (16 [interquartile range, 12-25] mm Hg) when compared with bioprostheses (28 [interquartile range, 18-41] mm Hg; mixed model P<0.001) and a similar degree of pulmonary regurgitation (degree 0-4) (1 [interquartile range, 0-2] versus 2 [interquartile range, 0-2]; mixed model P=0.19). At 9 years, freedom from structural valve deterioration and reintervention was 81.6% (95% CI, 71.5%-91.6%) versus 43.4% (95% CI, 23.6%-63.2%) in the homograft and bioprosthesis groups, respectively (adjusted hazard ratio, 0.27; 95% CI, 0.13-0.55; P<0.001). Conclusions When compared with bioprostheses, pulmonary homografts were associated lower transvalvular gradient during follow-up and were associated with a significantly lower risk of reintervention or structural valve degeneration.
Keywords: bioprosthesis; homograft; pulmonary heart disease; regurgitation; structural valve degeneration; tetralogy of Fallot.
Figures
References
-
- Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK. Long‐term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329:593–599. - PubMed
-
- Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. - PubMed
-
- Boethig D, Goerler H, Westhoff‐Bleck M, Ono M, Daiber A, Haverich A, Breymann T. Evaluation of 188 consecutive homografts implanted in pulmonary position after 20 years. Eur J Cardiothorac Surg. 2007;32:133–142. - PubMed
-
- Meijer FMM, Kies P, Jongbloed MRM, Hazekamp MG, Koolbergen DR, Blom NA, de Roos A, Schalij MJ, Vliegen HW. Excellent durability of homografts in pulmonary position analysed in a predefined adult group with tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2019;28:279–283. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

