Quantifying neurologic disease using biosensor measurements in-clinic and in free-living settings in multiple sclerosis
- PMID: 31840094
- PMCID: PMC6906296
- DOI: 10.1038/s41746-019-0197-7
Quantifying neurologic disease using biosensor measurements in-clinic and in free-living settings in multiple sclerosis
Abstract
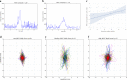
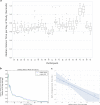
Technological advances in passive digital phenotyping present the opportunity to quantify neurological diseases using new approaches that may complement clinical assessments. Here, we studied multiple sclerosis (MS) as a model neurological disease for investigating physiometric and environmental signals. The objective of this study was to assess the feasibility and correlation of wearable biosensors with traditional clinical measures of disability both in clinic and in free-living in MS patients. This is a single site observational cohort study conducted at an academic neurological center specializing in MS. A cohort of 25 MS patients with varying disability scores were recruited. Patients were monitored in clinic while wearing biosensors at nine body locations at three separate visits. Biosensor-derived features including aspects of gait (stance time, turn angle, mean turn velocity) and balance were collected, along with standardized disability scores assessed by a neurologist. Participants also wore up to three sensors on the wrist, ankle, and sternum for 8 weeks as they went about their daily lives. The primary outcomes were feasibility, adherence, as well as correlation of biosensor-derived metrics with traditional neurologist-assessed clinical measures of disability. We used machine-learning algorithms to extract multiple features of motion and dexterity and correlated these measures with more traditional measures of neurological disability, including the expanded disability status scale (EDSS) and the MS functional composite-4 (MSFC-4). In free-living, sleep measures were additionally collected. Twenty-three subjects completed the first two of three in-clinic study visits and the 8-week free-living biosensor period. Several biosensor-derived features significantly correlated with EDSS and MSFC-4 scores derived at visit two, including mobility stance time with MSFC-4 z-score (Spearman correlation -0.546; p = 0.0070), several aspects of turning including turn angle (0.437; p = 0.0372), and maximum angular velocity (0.653; p = 0.0007). Similar correlations were observed at subsequent clinic visits, and in the free-living setting. We also found other passively collected signals, including measures of sleep, that correlated with disease severity. These findings demonstrate the feasibility of applying passive biosensor measurement techniques to monitor disability in MS patients both in clinic and in the free-living setting.
Keywords: Multiple sclerosis; Sensors and probes.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

