Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients
- PMID: 31840244
- PMCID: PMC6953317
- DOI: 10.1002/14651858.CD004290.pub3
Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients
Abstract
Background: Kidney transplantation is the therapy of choice for many patients with end-stage kidney disease (ESKD) with an improvement in survival rates and satisfactory short term graft survival. However, there has been little improvement in long-term survival. The place of target of rapamycin inhibitors (TOR-I) (sirolimus, everolimus), which have different modes of action from other commonly used immunosuppressive agents, in kidney transplantation remains uncertain. This is an update of a review first published in 2006.
Objectives: To evaluate the short and long-term benefits and harms of TOR-I (sirolimus and everolimus) when used in primary immunosuppressive regimens for kidney transplant recipients.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 20 September 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register were identified through searches of CENTRAL, MEDLINE and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: All randomised controlled trials (RCTs) and quasi-RCTs in which drug regimens, containing TOR-I commenced within seven days of transplant, were compared to alternative drug regimens, were included without age restriction, dosage or language of report.
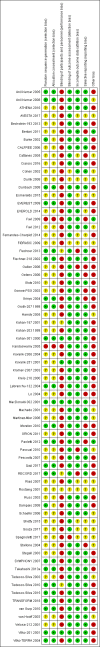
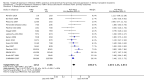
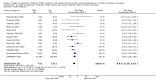
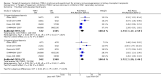
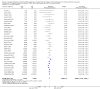
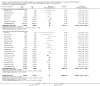
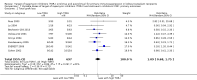
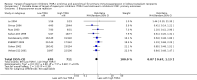
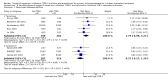
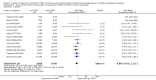
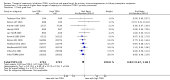
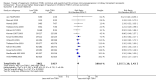
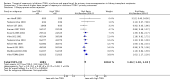
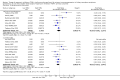
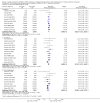
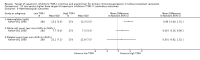
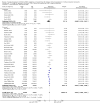
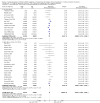
Data collection and analysis: Three authors independently assessed study eligibility, risk of bias, and extracted data. Results were reported as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous outcomes. Statistical analyses were performed using the random-effects model. The certainty of the evidence was assessed using GRADE MAIN RESULTS: Seventy studies (17,462 randomised participants) were included; eight studies included two comparisons to provide 78 comparisons. Outcomes were reported at six months to three years post transplant. Risk of bias was judged to be low for sequence generation in 25 studies, for allocation concealment in 23 studies, performance bias in four studies, detection bias in 65 studies, attrition bias in 45 studies, selective reporting bias in 48 studies, and for other potential bias in three studies. Risk of bias was judged to be at high risk of bias for sequence generation in two studies, allocation concealment in two studies, performance bias in 61 studies, detection bias in one study, attrition bias in four studies, for selective reporting bias in 11 studies and for other potential risk of bias in 46 studies. Compared with CNI and antimetabolite, TOR-I with antimetabolite probably makes little or no difference to death (RR 1.31, 95% CI 0.87 to 1.98; 19 studies) or malignancies (RR 0.86, 95% CI 0.50 to 1.48; 10 studies); probably increases graft loss censored for death (RR 1.32, 95% CI 0.96 to 1.81; 15 studies), biopsy-proven acute rejection (RR 1.60, 95% CI 1.25 to 2.04; 15 studies), need to change treatment (RR 2.42, 95% CI 1.88 to 3.11; 14 studies) and wound complications (RR 2.56, 95% CI 1.94 to 3.36; 12 studies) (moderate certainty evidence); but reduces CMV infection (RR 0.43, 95% CI 0.29 to 0.63; 13 studies) (high certainty evidence). Compared with antimetabolites and CNI, TOR-I with CNI probably makes little or no difference to death (RR 1.06, 95% CI 0.84 to 1.33; 31 studies), graft loss censored for death (RR 1.09, 95% CI 0.82 to 1.45; 26 studies), biopsy-proven acute rejection (RR 0.95, 95% CI 0.81 to 1.12; 24 studies); and malignancies (RR 0.83, 95% CI 0.64 to 1.07; 17 studies); probably increases the need to change treatment (RR 1.56, 95% CI 1.28 to 1.90; 25 studies), and wound complications (RR 1.56, 95% CI 1.28 to 1.91; 17 studies); but probably reduces CMV infection (RR 0.44, 95% CI 0.34 to 0.58; 25 studies) (moderate certainty evidence). Lower dose TOR-I and standard dose CNI compared with higher dose TOR-I and reduced dose CNI probably makes little or no difference to death (RR 1.07, 95% CI 0.64 to 1.78; 9 studies), graft loss censored for death (RR 1.09, 95% CI 0.54 to 2.20; 8 studies), biopsy-proven acute rejection (RR 0.87, 95% CI 0.67 to 1.13; 8 studies), and CMV infection (RR 1.42, 95% CI 0.78 to 2.60; 5 studies) (moderate certainty evidence); and may make little or no difference to wound complications (RR 0.95, 95% CI 0.53 to 1.71; 3 studies), malignancies (RR 1.04, 95% CI 0.36 to 3.04; 7 studies), and the need to change treatments (RR 1.18, 95% CI 0.58 to 2.42; 5 studies) (low certainty evidence). Lower dose of TOR-I compared with higher doses probably makes little or no difference to death (RR 0.84, 95% CI 0.67 to 1.06; 13 studies), graft loss censored for death (RR 0.92, 95% CI 0.71 to 1.19; 12 studies), biopsy-proven acute rejection (RR 1.26, 95% CI 1.10 to 1.43; 11 studies), CMV infection (RR 0.87, 95% CI 0.63 to 1.21; 9 studies), wound complications (RR 0.92, 95% CI 0.66 to 1.29; 7 studies), and malignancy (RR 0.84, 95% CI 0.54 to 1.32; 10 studies) (moderate certainty evidence); and may make little or no difference to the need to change treatments (RR 0.91, 95% CI 0.78 to 1.05; 10 studies) (low certainty evidence). It is uncertain whether sirolimus and everolimus differ in their effects on kidney function and lipid levels because the certainty of the evidence is very low based on a single small study with only three months of follow-up.
Authors' conclusions: In studies with follow-up to three years, TOR-I with an antimetabolite increases the risk of graft loss and acute rejection compared with CNI and an antimetabolite. TOR-I with CNI potentially offers an alternative to an antimetabolite with CNI as rates of graft loss and acute rejection are similar between interventions and TOR-I regimens are associated with a reduced risk of CMV infections. Wound complications and the need to change immunosuppressive medications are higher with TOR-I regimens. While further new studies are not required, longer-term follow-up data from participants in existing methodologically robust RCTs are needed to determine how useful immunosuppressive regimens, which include TOR-I, are in maintaining kidney transplant function and survival beyond three years.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
None known.
Figures





















































































Update of
-
Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD004290. doi: 10.1002/14651858.CD004290.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 Dec 16;12:CD004290. doi: 10.1002/14651858.CD004290.pub3. PMID: 16625599 Updated.
References
References to studies included in this review
Anil Kumar 2005 {published and unpublished data}
-
- Anil Kumar MS, Fyfe B, Sierka D, Heifets M, Saeed MI, Parikh MH. Comparison of efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) as adjunct to calcineurin inhibitor (CNI) based steroid free immunosuppression in kidney transplantation [abstract no: 1530]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509056]
-
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80(6):807‐14. [MEDLINE: ] - PubMed
-
- Anil Kumar MS, Heifets M, Fyfe B, Sierka D, Saeed MI, Parekh M, et al. A prospective randomized study to compare the efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) monitored by protocol biopsies in tacrolimus (TAC) based steroid free immunosuppression [abstract no: 212]. American Journal of Transplantation 2004;4(Suppl 8):216. [CENTRAL: CN‐00509296]
-
- Kumar A, Lee D, Xiao SG, Moritz MJ, Fyfe B, Heifets M, et al. Comparison of tacrolimus (FK506) and sirolimus (SRL) combination with FK506 and mycophenolate mofetil (MMF) in kidney transplant recipients with steroid avoidance [abstract no: 771]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446223]
Anil Kumar 2008 {published data only}
-
- Anil Kumar MS, Heifets M, Fyfe B, Moritz MJ, Saeed MI, Parikh MH, et al. Comparison of four different steroid avoidance protocols in kidney transplantation: tacrolimus (TAC)/mycophenolate mofetil (MMF) versus Tac/sirolimus (SLR) versus cyclosporine (CSA)/MMF versus CSA/SLR [abstract no: 512]. American Journal of Transplantation 2005;5(Suppl 11):286‐7. [CENTRAL: CN‐00679049]
-
- Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento‐Reodica N, Kumar AM, et al. Comparison of four different immunosuppression protocols without long‐term steroid therapy in kidney recipients monitored by surveillance biopsy: five‐year outcomes. Transplant Immunology 2008;20(1‐2):32‐42. [MEDLINE: ] - PubMed
ATHENA 2016 {published data only}
-
- Dragun D, Sommerer C, Hauser IA, Suwelack BM, Schenker P, Witzke O, et al. Everolimus [EVR]‐based versus tacrolimus [TAC]‐MPA regimen in de novo kidney transplant recipients: 12 months safety and efficacy data from the ATHENA study [abstract no: SA‐PO510]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):810. [CENTRAL: CN‐01657414]
-
- Dragun D, Sommerer C, Suwelack B, Schenker P, Hauser I, Witzke O, et al. ATHENA study outcomes on allograft function after 12 months with everolimus‐CNI vs tacrolimus‐MPA regimen in de novo renal transplant recipients [abstract no: PLB099]. Transplant International 2017;30(Suppl 2):562. [EMBASE: 618769585]
-
- Dragun D, Suwelack B, Sommerer C, Hauser IA, Witzke O, Hugo C, et al. Significant anti‐CMV/BKV effect of a modern everolimus‐based regimen comparted to standard tacrolimus‐MPA regimen in De novo kidney transplant recipients: ATHENA 12 months data on infections [abstract no: 401.2]. Transplantation 2018;102(7 Suppl 1):S128. [EMBASE: 623701445]
-
- Hauser A, Sommerer C, Suwelack B, Dragun D, Witzke O, Hugo C, et al. Significant anti‐CMV/BKV effect of a modern everolimus‐based regimen comparted to a standard tacrolimus‐MPA regimen in de novo kidney transplant recipients: ATHENA 12 months data on infections [abstract no: 183]. American Journal of Transplantation 2018;18(Suppl 4):318. [EMBASE: 622280450]
-
- Hauser IA, Marx S, Sommerer C, Suwelack B, Dragun D, Witzke O, et al. Differences in CMV‐specific CD4 T cell population in de novo kidney transplant recipients treated with everolimus‐based regimen compared to a standard tacrolimus‐MPA regimen: Results from ATHENA [abstract]. American Journal of Transplantation 2019;19(Suppl 3):520. [EMBASE: 628454558]
AVESTA 2017 {published data only}
-
- Gholi FP, Dalili N, Alinezhad M, Moosavi M. AVESTA study‐an Iranian verification of everolimus success in transplantation aspects [abstract no: SP779]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii406‐7. [EMBASE: 617290880]
Bechstein‐193 2013 {published data only}
-
- Bechstein W, Paczek L, European Rapamune‐FK Study Group. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 1321]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415252]
-
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, Zygmunt AJ, European Rapamune Tacrolimus Study Group. A comparative, randomized trial of concentration‐controlled sirolimus combined with reduced‐dose tacrolimus or standard‐dose tacrolimus in renal allograft recipients. Transplantation Proceedings 2013;45(6):2133‐40. [MEDLINE: ] - PubMed
-
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus + steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract no: W739]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00444369]
-
- Paczek L, Bechstein W, Wramner L, Squifflet JP. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 170]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, USA. 2002. [CENTRAL: CN‐01912222]
-
- Paczek L, Bechstein WO, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus +steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract no: 1219]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐01912354]
Bertoni 2011 {published data only}
-
- Bertoni E, Becherelli P, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolate acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. a monocentric experience [abstract no: F‐PO614]. Journal of the American Society of Nephrology 2007;18(Abstracts):234A. [CENTRAL: CN‐00716078]
-
- Bertoni E, Larti A, Farsetti S, Rosso G, Maria L, Zanazzi M. Cyclosporine (CyA) very low dose with everolimus (E) high dose is associated with better outcomes in renal transplant patients with respect to standard treatment with EC‐MPS (M) [abstract no: LB‐1]. Transplant International 2009;22(Suppl 2):91. [CENTRAL: CN‐01657256]
-
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. Journal of Nephrology 2011;24(5):613‐8. [MEDLINE: ] - PubMed
-
- Rosati A, Bertoni E, Rosso G, Larti A, Mehmetaj A, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolic acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. a monocentric experience [abstract no: SaP477]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi396. [CENTRAL: CN‐00725011]
Burke 2002 {published data only}
-
- Burke GW, Ciancio C, Blomberg BB, Rosen A, Suzart K, Roth D, et al. Randomized trial of three different immunosuppressive regimens to prevent chronic renal allograft rejection. Transplantation Proceedings 2002;34(5):1610‐1. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77(2):244‐51. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation 2004;77(2):252‐8. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Gaynor JJ, Ruiz P, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three‐year analysis. Transplantation 2006;81(6):845‐52. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Mattiazzi AD, Gaynor JJ, Roohipour R, Dowdy L, et al. Prophylaxis for cytomegalovirus disease in a randomized trial of three different immunosuppressive regimens in renal transplantation [abstract no: 1228]. American Journal of Transplantation 2004;4(Suppl 8):494. [CENTRAL: CN‐00509136]
CALFREE 2006 {published data only}
-
- Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus and cyclosporine based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. American Journal of Kidney Diseases 2010;55(2):335‐43. [MEDLINE: ] - PubMed
-
- Franz SE, Bengue O, Dickenmann MJ, Brunner F, Steiger J. Tubulotoxicity of sirolimus [abstract no: O60]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):19. [CENTRAL: CN‐00509200]
-
- Franz SE, Regeniter A, Mihatsch M, Steiger JU. Tubulotoxic properties of sirolimus [abstract no: F‐FC0057]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):12A. [CENTRAL: CN‐00445379]
-
- Giannini O, Dickenmann M, Kim MJ, Franz S, Mayr M, Mihatsch MJ, et al. The CALFREE Study ‐ an open, prospective, randomized single center study to investigate calcineurin free immunosuppression in 100 de novo, normal risk renal transplant recipients: preliminary results [abstract no: P04.02]. Kidney & Blood Pressure Research 2004;27(5‐6):329. [CENTRAL: CN‐00615861]
-
- Kim MJ, Dickenmann MJ, Moll S, Mihatsch MJ, Brunner F, Steiger J. Protocol biopsies: do they improve patient management? [abstract no: T466]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):317. [CENTRAL: CN‐00509278]
Cattaneo 2005 {published data only}
-
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, et al. Influence of co‐medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. American Journal of Transplantation 2005;5(12):2937‐44. [MEDLINE: ] - PubMed
-
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Perico N, Gotti E, et al. Effects of sirolimus on the pharmacokinetics of mycophenolic acid in kidney transplantation [abstract no: 650]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐00724878] - PubMed
-
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. Journal of the American Society of Nephrology 2007;18(3):1007‐18. [MEDLINE: ] - PubMed
-
- Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84(8):956‐64. [MEDLINE: ] - PubMed
-
- Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low‐dose rabbit anti‐thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor‐specific anti‐HLA antibody development. Journal of Immunology 2013;191(5):2818‐28. [MEDLINE: ] - PubMed
Ciancio 2016 {published data only}
-
- Ciancio G, Tryphonopoulos P, Gaynor JJ, Guerra G, Sageshima J, Roth D, et al. Pilot randomized trial of tacrolimus/everolimus vs tacrolimus/enteric‐coated mycophenolate sodium in adult, primary kidney transplant recipients at a single center. Transplantation Proceedings 2016;48(6):2006‐10. [MEDLINE: ] - PubMed
Cohen 2002 {published data only}
-
- Cohen DJ, Vincenti F. A comparative, open‐label study to evaluate graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids. [abstract no: 394]. American Journal of Transplantation 2002;2(Suppl 3):237. [CENTRAL: CN‐01912453]
-
- Cohen DJ, Vincenti F, US Rapamune‐CSA Study Group. A comparative, open‐label study to evalute graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids [abstract no: 1223]. American Journal of Transplantation 2003;3(Suppl 5):465. [CENTRAL: CN‐00444868]
-
- Vincenti F, Cohen DJ. A comparative, open‐label study to evaluate graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00420865]
Durlik 2008 {published data only}
-
- Durlik M, Tronina O, Baczkowska T, Klinger M, Rutkowski B, Wiecek A, et al. Sirolimus is associated with worsen outcome compared to mycophenolate mofetil in high immunologic risk renal transplant recipients receiving tacrolimus combined with ATG induction [abstract no: 533]. Transplantation 2008;86(Suppl 2):186. [CENTRAL: CN‐00740488]
Durrbach 2008 {published data only}
-
- Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, Kessler M, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys [abstract no: P102]. Transplantation 2004;78(2 Suppl):228.
-
- Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation 2008;85(3):486‐90. [MEDLINE: ] - PubMed
-
- Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys [abstract no: 686]. American Journal of Transplantation 2004;4(Suppl 8):345. [CENTRAL: CN‐00509509]
-
- Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Use of sirolimus as initial therapy in renal transplant recipients of marginal kidneys [abstract no: F‐PO1051]. Journal of the American Society of Nephrology 2004;15(Oct):295A. [CENTRAL: CN‐00583227]
Esmeraldo 2015 {published data only}
-
- Esmeraldo R, Oliveira M, Pinheiro P, Girao C, Freitas T. Prospective randomized open trial designed to reduce the incidence of cytomegalovirus (CMV) infection in de novo kidney transplant recipients two‐year results [abstract no: 39]. American Journal of Transplantation 2016;16(Suppl 3):218. [EMBASE: 611700413]
-
- Esmeraldo R, Oliveira MLM, Pinheiro PM, Girao CM. One‐year results of a prospective randomized open trial designed to reduce the incidence of cytomegalovirus (CMV) infection in de novo kidney transplant recipients [abstract no: O05]. Transplant International 2015;28(Suppl 4):2. [EMBASE: 72111250]
-
- Esmeraldo RM, Sandes‐Freitas TM, Girao CM, Oliveira‐Sales ML, Pinheiro PM. Everolimus enables tacrolimus and steroid minimization with reduced incidence of CMV infection and preserved graft function in de novo renal transplant recipients at 12 and 24 months post‐transplant [abstract no: OS164]. Transplant International 2017;30(Suppl 2):64. [EMBASE: 618769705]
EVEREST 2009 {published data only}
-
- Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the Everest Study [abstract no: P118]. Transplant International 2007;20(Suppl 2):124. [CENTRAL: CN‐00724884]
-
- Corbetta G, Salvadori M, Scolari MP, Citterio F, Rigotti P, Cossu M, et al. Exposure to everolimus, and not to cyclosporine, is associated with freedom from acute rejection in de novo renal recipients [abstract no: 447]. Transplantation 2008;86(Suppl 2):157. [CENTRAL: CN‐00740485]
-
- Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, et al. Everolimus and minimization of cyclosporine in renal transplantation: 24‐month follow‐up of the EVEREST study. Transplantation 2011;91(10):e72‐3. [MEDLINE: ] - PubMed
-
- Salvadori M, Scolari M, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Targeting upper everolimus blood levels with very low‐dose cyclosporine is effective and safe in de novo renal transplantation [abstract no: 448]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740453]
-
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M. Upper everolimus blood levels with very low‐dose cyclosporin: 12 months follow up of the Everest Study [abstract no: 1085]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00776867]
EVEROLD 2014 {published data only}
-
- Meur Y, Buchler M, Mousson C, Moal M, Albano L, Merville P, et al. EVEROLD: a multicenter randomized study for the use of everolimus in "old‐for‐old" renal transplantation [abstract no: 2155]. Transplantation 2014;98(Suppl 1):114. [EMBASE: 71543890]
Favi 2009 {published data only}
-
- Favi E, Citterio F, Spagnoletti G, Gargiulo A, Delreno F, Romagnoli J, et al. Prospective clinical trial comparing two immunosuppressive regimens, tacrolimus and mycophenolate mofetil versus everolimus and low‐dose cyclosporine, in de novo renal transplant recipients: results at 6 months follow‐up. Transplantation Proceedings 2009;41(4):1152‐5. [EMBASE: 2009227250] - PubMed
-
- Favi E, Citterio F, Spagnoletti G, Romagnoli J, Gargiulo A, Castagneto M. Tacrolimus and MMF vs everolimus and low dose cyclosporine: 2 year results of a prospective clinical trial [abstract no: Su665]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00784295]
-
- Favi E, Citterio F, Spagnoletti G, Tondolo V, Romagnoli J, Castagneto M. A prospective trial comparing tacrolimus with everolimus and low dose cyclosporine [abstract no: 1155]. American Journal of Transplantation 2007;7(Suppl 2):443. [CENTRAL: CN‐00653736]
-
- Favi E, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M, Citterio F. The combination of everolimus and low dose cyclosporine allows similar results as the standard tacrolimus and MMF regimen: 3 year results of a prospective clinical trial in renal transplant recipients [abstract no: 1645]. American Journal of Transplantation 2010;10(Suppl 4):506. [CENTRAL: CN‐00776468]
-
- Favi E, Spagnoletti G, Salerno MP, Pedroso JA, Romagnoli J, Citterio F. Tacrolimus plus mycophenolate mofetil vs. cyclosporine plus everolimus in deceased donor kidney transplant recipients: three‐yr results of a single‐center prospective clinical trial. Clinical Transplantation 2013;27(4):E359‐67. [MEDLINE: ] - PubMed
Favi 2012 {published data only}
-
- Favi E, Silvestrini N, Pedroso J, Salerno M, Spagnoletti G, Bianchi V, et al. Extended‐release tacrolimus plus everolimus vs extended‐release tacrolimus plus micophenolate mofetil in primary deceased donor kidney transplant recipients: 1‐year results of an open label, randomized phase 2 clinical trial [abstract no: B950]. American Journal of Transplantation 2013;13(Suppl 5):316. [EMBASE: 71057526]
-
- Favi E, Silvestrini N, Pedroso JA, Salerno MP, Spagnoletti G, Romagnoli J, et al. ER‐tacrolimus plus everolimus vs ER‐tacrolimus plus MMF in primary deceased donor kidney transplantation: 1‐year results of single center, open label, prospective, randomized clinical trial [abstract no: P288]. Transplant International 2013;26(Suppl 2):241. [EMBASE: 71359898]
-
- Favi E, Silvestrini N, Salerno MP, Romagnoli J, Citterio F. Extended‐release tacrolimus plus everolimus or micophenolate mofetil in deceased donor kidney transplant recipients: 6‐month results of a prospective randomized clinical trial [abstract no: 55]. American Journal of Transplantation 2012;12(Suppl 3):42‐3. [EMBASE: 70746002]
Fernandes‐Charpiot 2014 {published data only}
-
- Abbud‐Filho M, Mazeti C, Caldas H, Fernandes I, Razera J, Baptista MA. Identifying the cytokine molecular profile in renal transplant recipients of extended criteria donor kidneys [abstract no: P461]. Transplant International 2015;28(Suppl 4):590. [EMBASE: 72112425]
-
- Fernandes‐Charpiot I, Caldas H, Mazeti C, Baptista M, Razera J, Abbud‐Filho M. Efficacy and safety of early use of everolimus in standard and extended criteria deceased donor kidney transplant recipients: 12‐month preliminary results of a randomized single center trial [abstract no: C1855]. Transplantation 2014;98(Suppl 1):597‐8. [EMBASE: 71545554]
FIBRASIC 2009 {published data only}
-
- Woestenburg AT, Peeters P, Sennesael J, Abramowicz D, Wissing KM, Geers C, et al. Interstitial fibrosis and fibrous intimal thickening in de novo renal allografts under sirolimus or cyclosporine: results of a randomised, controlled trial (FIBRASIC) [abstract no: O‐300]. Transplant International 2009;22(Suppl 2):79. [CENTRAL: CN‐01658203]
Flechner 2013 {published data only}
-
- Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open‐label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 2013;95(10):1233‐41. [MEDLINE: ] - PubMed
-
- Flechner SM, Gurkan A, See Tai S, Schulman SL. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based versus cyclosporine (CsA)‐based regimen in de novo renal allograft recipients [abstract no: 300]. American Journal of Transplantation 2009;9(Suppl 2):278. [CENTRAL: CN‐00775180]
Flechner‐318 2002 {published data only}
-
- Campistol JM, Glyda M, Gurkan A, Flechner SM, Schulman S, See Tai S. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based regimens compared with calcineurin inhibitors (CNIs) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: P‐105]. Transplant International 2009;22(Suppl 2):121. [CENTRAL: CN‐01657955]
-
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract no: 1164]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351]
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract no: 0378]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415657]
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches. [abstract no: 1317]. American Journal of Transplantation 2002;2(Suppl 3):470. [CENTRAL: CN‐01912448]
-
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] - PubMed
Gallon 2006 {published and unpublished data}
-
- Chhabra D, Skaro AI, Leventhal JR, Dalal P, Shah G, Wang E, et al. Long‐term kidney allograft function and survival in prednisone‐free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(3):504‐12. [MEDLINE: ] - PMC - PubMed
-
- Dalal P, Xu L, Joseph L, Shah G, Chhabra D, Wang E, et al. Prospective randomized study to evaluate the long term impact on graft survival and function of two pred‐free, CNI based maintenance immunosuppression: FK/MMF vs. FK/SRL [abstract no: 1670]. American Journal of Transplantation 2010;10(Suppl 4):512. [CENTRAL: CN‐00775927]
-
- Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, et al. Long‐term renal allograft function on a tacrolimus‐based, pred‐free maintenance immunosuppression comparing sirolimus vs MMF. American Journal of Transplantation 2006;6(7):1617‐23. [MEDLINE: ] - PubMed
-
- Gallon L, Perico N, Winoto J, Dimitrov B, Leventhal J, Gaspari F, et al. Prospective randomized single center study comparing the impact on long term renal transplant function of two prednisone free maintenance immunosuppressive combinations with tacrolimus (TAC), mycophenolate mofetil (MMF) versus tacrolimus/sirolimus (SLR) [abstract no: 517]. American Journal of Transplantation 2005;5(Suppl 11):288. [CENTRAL: CN‐00583215]
-
- Gallon LG, Kaufman DB, Leventhal JR, Abecassis M, Fryer J, Koffron A, et al. A prospective randomized single center study of prednisone free immunosuppression that compares two maintenance combinations: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. [abstract no: SU‐PO598]. Journal of the American Society of Nephrology 2003;14(Nov):664A. [CENTRAL: CN‐00583857]
Gelens 2006 {published data only}
-
- Gelens M, Christiaans M, Hooff J. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: P‐3]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
-
- Gelens M, Christiaans M, Hooff J. Incidence of post transplant diabetes mellitus during calcineurin‐free and calcineurin‐based immunosuppression with limited steroid exposure [abstract no: 1210]. American Journal of Transplantation 2005;5(Suppl 11):465. [CENTRAL: CN‐01657297]
-
- Gelens M, Christianns M, Heurn E, Hooff J. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: 865]. American Journal of Transplantation 2005;5(Suppl 11):376. [CENTRAL: CN‐01657298]
-
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] - PubMed
Glotz 2010 {published data only}
-
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. 6 months preliminary results of a randomized trial comparing sirolimus (SRL) versus tacrolimus (FK) in 141 transplant patients receiving a cadaveric renal graft [abstract no: 1191]. American Journal of Transplantation 2005;5(Suppl 11):460. [CENTRAL: CN‐00583237]
-
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89(12):1511‐7. [MEDLINE: ] - PubMed
Gonwa‐PSG 2003 {published data only}
-
- Fitzsimmons WE, First MR, Gao J. Correlation of tacrolimus trough blood levels to rejection and adverse events after kidney transplantation [abstract no: 649]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐01657974]
-
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. One year results for the first prospective multi‐center kidney transplant study comparing tacrolimus+sirolimus vs. tacrolimus+MMF combination therapy [abstract no: P202]. Transplantation 2004;78(2 Suppl):263‐4. [CENTRAL: CN‐00509213]
-
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S, et al. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75(8):1213‐20. [MEDLINE: ] - PubMed
-
- Gonwa T, Rice K, Langnas A, Tomlandovich S, Danovitch G, Inokuchi S, et al. Sirolimus vs MMF‐first report of a US multicenter kidney transplant study with tacrolimus combination therapy [abstract no: 1320]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415747]
-
- Gonwa TA, Fujisawa Study Group. Final results for the first prospective, multi‐center kidney transplant study comparing tacrolimus +sirolimus vs tacrolimus+MMF combination therapy [abstract no: F‐FC040]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):9‐10A. [CENTRAL: CN‐00550649]
Grinyo 2004 {published data only}
-
- Campistol JM, Grinyo JM, Paul J, Garcia J, Arias M, Morales JM, et al. Sirolimus, TAC and corticosteroids in the postoperative period. Preliminary results [abstract no: 2108]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415380]
-
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract no: SA‐PO0491]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):363A. [CENTRAL: CN‐00445561]
-
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):101.
-
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (TAC) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, tac and corticosteroids in the postoperative period. Preliminary results [abstract no: 1018]. American Journal of Transplantation 2002;2(Suppl 3):394. [CENTRAL: CN‐00415775]
-
- Grinyo JM, Campistol JM, Paul J, Garcia‐Martinez J, Morales JM, Prats D, et al. Pilot randomized study of early tacrolimus withdrawal from a regimen with sirolimus plus tacrolimus in kidney transplantation. American Journal of Transplantation 2004;4(8):1308‐14. [MEDLINE: ] - PubMed
Groth‐207 1999 {published data only}
-
- Campistol JM, Backman L, Brattstrom C, Kreis H, Morales JM, Sirolimus European Renal Transplant Study Group. Influence of immunosupressive treatment on markers of bone remodeling in renal transplantation: comparison between cyclosporine and sirolimus [abstract no: A3814]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):754A. [CENTRAL: CN‐00550450]
-
- Campistol JM, Holt DW, Epstein S, Gioud‐Paquet M, Rutault K, Burke JT, et al. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transplant International 2005;18(9):1028‐35. [MEDLINE: ] - PubMed
-
- Charpentier B, Groth CG, Backman L, Morales JM, Calne R, Kreis H, et al. Bicentre hospital experience with sirolimus‐based therapy in human renal transplantation: the Sirolimus European Renal Transplant Study. Transplantation Proceedings 2003;35(3 Suppl):58S‐61S. [MEDLINE: ] - PubMed
-
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)‐based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999;67(7):1036‐42. [MEDLINE: ] - PubMed
-
- Groth CG, Brattstrom C, Claesson K, Backman L. New trails in transplantation: how to exploit the potential of sirolimus in clinical transplantation. Transplantation Proceedings 1998;30(8):4064‐5. [MEDLINE: ] - PubMed
Hamdy 2005 {published data only}
-
- Hamdy A, El‐Baz M, Bakr M, Ghoneim M. The incidence of chronic allograft nephropathy among live‐donor renal transplant recipients primarily treated with sirolimus‐based regimens [abstract no: Sa734]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776624]
-
- Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live‐donor renal transplant recipients' long‐term experience. Experimental & Clinical Transplantation 2010;8(4):283‐91. [MEDLINE: ] - PubMed
-
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy El‐M, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] - PubMed
-
- Hamdy AF, Elhadedy MA, Donia AF, Taha NM, Bakr MA. Outcome of sirolimus‐based immunosuppression, fifteen years post‐live‐donor kidney transplantation: single‐center experience. Clinical Transplantation 2019;33(2):e13463. [MEDLINE: ] - PubMed
Kahan‐157 2001 {published data only}
-
- Kahan BD, Kaplan B, Lorber M, Winkler M, Cambon N, Maca J, et al. Multicenter, randomized, double‐blind, dose‐finding study evaluating the efficacy and safety of RAD001 (RAD) in de novo renal transplant recipients [abstract no: 954]. Transplantation 2000;69(8 Suppl):S359. [CENTRAL: CN‐00445976]
-
- Kahan BD, Kaplan B, Lorber M, Winkler M, Campbell M, Maca J, et al. Safety and preliminary efficacy of RAD in de novo renal transplant recipients evaluated in a multicenter, randomized, double‐blind, dose‐finding study [abstract no: 0662]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445977]
-
- Kahan BD, Kaplan B, Lorber MI, Winkler M, Cambon N, Boger RS. RAD in de novo renal transplantation: comparison of three doses on the incidence and severity of acute rejection. Transplantation 2001;71(10):1400‐6. [MEDLINE: ] - PubMed
-
- Kovarik JM, Kaplan B, Vitko S, McMahon L, Attinger M, Boger R, et al. Longitudinal influence of everolimus on cyclosporine assessed over 6 months in two blinded de novo kidney transplant trials [abstract no: 1341]. American Journal of Transplantation 2001;1(Suppl 1):475. [CENTRAL: CN‐00583198]
-
- Kovarik JM, Rordorf C, Kahan BD, Kaplan B, Lorber M, Winkler M, et al. Longitudinal assessment of the steady‐state pharmacokinetics of RAD in de novo renal transplant patients [abstract no: 160]. Transplantation 2000;69(8 Suppl):S154. [CENTRAL: CN‐00446175]
Kahan‐203 1999 {published data only}
-
- Groth CG, Brattstrom C, Claesson K, Backman L. New trails in transplantation: how to exploit the potential of sirolimus in clinical transplantation. Transplantation Proceedings 1998;30(8):4064‐5. [MEDLINE: ] - PubMed
-
- Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in Caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation 1999;68(10):1526‐32. [MEDLINE: ] - PubMed
-
- Kahan BD, Kramer WG. Median effect analysis of efficacy versus adverse effects of immunosuppressants. Clinical Pharmacology & Therapeutics 2001;70(1):74‐81. [MEDLINE: ] - PubMed
Kahan‐301 2000 {published data only}
-
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. American Journal of Transplantation 2002;2(6):551‐9. [MEDLINE: ] - PubMed
-
- Blum CB, Rapamune US Study Group. Cholesterol and triglyceride levels in sirolimus‐treated renal transplant recipients [abstract no: A3577]. Journal of the American Society of Nephrology 2000;11(Sept):680A. [CENTRAL: CN‐00550429]
-
- Campistol JM, Cole E, Gioud‐Paquet M, US European and Global Renal Transplant Study Groups. Sirolimus (rapamune): effects on bone in renal transplant patients [abstract no: 681]. Transplantation 2000;69(8 Suppl):S289. [CENTRAL: CN‐00444659]
-
- Campistol JM, Legendre C, Castegneto M, Hartmann A, European US Global and Tri‐Continental Study Groups. The effect of sirolimus on lipids on renal transplant recipients: results from clinical trials [abstract no: A4606]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):880A. [CENTRAL: CN‐00550752]
-
- Campistol JM, Legendre C, Hartmann A, Castagneto M, European US Global and Tri‐Continental Study Group. Lipid effects in renal allograft recipients receiving sirolimus‐based therapies [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00444660]
Kandaswamy 2005 {published data only}
-
- Kandaswamy R, Humar A, Dunn T, Gross E, Hughes M, Hill M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: 5‐year results [abstract no: 286]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00677752]
-
- Kandaswamy R, Humar A, Khwaja K, Asolati M, Harmon J, Gillingham K, et al. A prospective randomized study of cyclosporine (CsA)/Cellcept (MMF) vs. tacrolimus (TAC)/sirolimus (SIR) with rapid discontinuation of prednisone (P) [abstract no: 182]. American Journal of Transplantation 2003;3(Suppl 5):198. [CENTRAL: CN‐00445992]
-
- Kandaswamy R, Humar A, Sturdevant M, Garcia‐Roca R, Casingal V, Tan M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: mixed results [abstract no: 329]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00766092]
-
- Kandaswamy R, Humar A, Sutherland DE, Gillingham K, Matas A. A prospective, randomized study of cyclosporine (CsA)/mycophenolate mofetil (MMF) versus tacrolimus (TAC)/sirolimus(SIR) with rapid discontinuation of prednisone (P) [abstract no: 084]. Transplantation 2004;78(2 Suppl):32. [CENTRAL: CN‐00509261]
-
- Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid‐free maintenance regimens in kidney transplant recipients‐‐an interim analysis. American Journal of Transplantation 2005;5(6):1529‐36. [MEDLINE: ] - PubMed
Kovarik‐2306 2004 {published data only}
-
- Kovarik JM, Tedesco H, Pascual J, Civati G, Bizot MN, Geissler J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced‐exposure cyclosporine in de novo kidney transplantation. Therapeutic Drug Monitoring 2004;26(5):499‐505. [MEDLINE: ] - PubMed
-
- Kovarik JM, Tedesco H, Pascual J, Civati G, Schmidli H, Geissler J. Everolimus therapeutic concentration range derived from prospective concentration‐controlled trial in de novo kidney transplantation [abstract no: 510]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509289] - PubMed
-
- Magee J, Tedesco H, Pascual J, Civati G, Filho G, Garcia V, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral® in de novo kidney transplant recipients: 12 months analysis [abstract no: 504]. American Journal of Transplantation 2004;4(Suppl 8):296‐7. [CENTRAL: CN‐00509335]
-
- Pascual J. Concentration‐controlled everolimus (Certican): combination with reduced dose calcineurin inhibitors. Transplantation 2005;79(9 Suppl):S76‐9. [MEDLINE: ] - PubMed
-
- Pascual J, Cambi V, Dissegna D, Esmeraldo R, Lao M, Durlik M, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose Neoral® in de novo kidney transplant recipients: 24 months analysis [abstract no: 1010]. American Journal of Transplantation 2005;5(Suppl 11):414. [CENTRAL: CN‐00725012]
Kovarik‐251 2001 {published data only}
-
- Kaplan B, Tedesco‐Silva H, Mendez R, Kahan B, Buren D, Boger R, et al. North/South American, double‐blind, parallel group study of the safety and efficacy of Certican (RAD) versus mycophenolate mofetil (MMF) in combination with neoral and corticosteroids [abstract no: 1339]. American Journal of Transplantation 2001;1(Suppl 1):475. [CENTRAL: CN‐00446004]
-
- Kaplan B, Tedesco‐Silva H, Mendez R, Kahan BD, Buren D, Boger R, et al. Everolimus (RAD) ‐ 12 month pivotal study results; the efficacy and safety in conjunction with neoral® and steroids [abstract no: A4700]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):899A. [CENTRAL: CN‐00583195]
-
- Kovarik JM, Hartmann S, Berthier S, Hubert M, Rordorf C, Somberg K. Statin therapy in renal transplant patients receiving everolimus: screening for clinical and pharmacokinetic drug interactions [abstract no: 187]. American Journal of Transplantation 2002;2(Suppl 3):185. [CENTRAL: CN‐00416055]
-
- Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clinical Pharmacology & Therapeutics 2001;70(3):247‐54. [MEDLINE: ] - PubMed
-
- Kovarik JM, Kaplan B, Silva HT, Kahan BD, Dantal J, McMahon L, et al. Pharmacokinetics of an everolimus‐cyclosporine immunosuppressive regimen over the first 6 months after kidney transplantation. American Journal of Transplantation 2003;3(5):606‐13. [MEDLINE: ] - PubMed
Kramer‐2307 2003 {published data only}
-
- Campbell S, Eris J, Brown F, Russ G, Caicedo L, Walker R, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 24 month result [abstract no: FC‐50002]. Nephrology 2005;10(Suppl 1):A1. [CENTRAL: CN‐01912374]
-
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. 36 month result showing excellent graft function in de novo kidney transplant recipients treated with everolimus, basiliximab and reduced cyclosoporin exposure [abstract]. Immunology & Cell Biology 2007;85(4):A35.
-
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CsA and basiliximab at 24 months [abstract no: 36]. Transplantation Society of Australia & New Zealand (TSANZ). 24th Annual Scientific Meeting; 2006 Mar 29‐31; Canberra, Australia. 2006:53. [CENTRAL: CN‐00583469]
-
- Eris J, Campbell S, Burbigott B, Leone J, Kraemer B, Rigotti P, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced neoral® exposure: 12‐month results [abstract no: O82]. Transplantation 2004;78(2 Suppl):31. [CENTRAL: CN‐01912456]
-
- Eris J, Campbell S, Walker R, Russ G, Stambe C, RAD2307 International Study Group. Excellent graft function in de novo transplant recipients treated with everolimus, reduced dose Neoral and Simulect: 6 months analysis [abstract no: 5]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:33. [CENTRAL: CN‐00509178]
Kreis‐210 2000 {published data only}
-
- Abramowicz D, Kreis H, Campistol JM, Morales JM, Mourad G, Holt DW, et al. Prednisolone pharmacokinetics in sirolimus ‐ and cyclosporine ‐ treated renal transplant recipients [abstract no: PO551]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00444096]
-
- Campistol JM, Holt DW, Epstein S, Gioud‐Paquet M, Rutault K, Burke JT, et al. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transplant International 2005;18(9):1028‐35. [MEDLINE: ] - PubMed
-
- Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000;69(7):1252‐60. [MEDLINE: ] - PubMed
-
- Kreis H, Cisterne JM, Land W, Wramner L, Sirolimus European Renal Transplant Study Group. Sirolimus versus cyclosporine in association with mycophenolate mofetil [abstract no: 959]. Transplantation 2000;69(8 Suppl):360. [CENTRAL: CN‐00446198]
-
- Legendre C, Campistol JM, Squifflet JP, Burke JT, Sirolimus European Renal Transplant Study Group. Cardiovascular risk factors of sirolimus compared with cyclosporine: Early experience from two randomized trials in renal transplantation. Transplantation Proceedings 2003;35(3 Suppl):151‐3S. [MEDLINE: ] - PubMed
Lebranchu‐132 2004 {published and unpublished data}
-
- Al Najjar A, Etienne I, Toupance O, Westeel PF, Hurault de Ligny B, Rerolle JP, et al. Long term follow‐up of a multicenter randomized trial comparing a CNI‐free regimen with sirolimus (SRL) to a cyclosporine based regimen: the Spiesser study [abstract no: 1642]. American Journal of Transplantation 2010;10(Suppl 4):505. [EMBASE: 70465017]
-
- Buchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6‐month course of steroids. American Journal of Transplantation 2007;7(11):2522‐31. [MEDLINE: ] - PubMed
-
- Buchler M, Lebranchu Y, Beneton M, Meur Y, Heng AE, Westeel PF, et al. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine cotreatment. Clinical Pharmacology & Therapeutics 2005;78(1):34‐42. [MEDLINE: ] - PubMed
-
- Gatault P, Bertrand D, Buchler M, Colosio C, Hurault de Ligny B, Weestel PF, et al. Eight‐year results of the Spiesser study, a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation. Transplant International 2016;29(1):41‐50. [MEDLINE: ] - PubMed
-
- Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Bellien J, et al. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transplant International 2010;23(11):1135‐43. [MEDLINE: ] - PubMed
Lo 2004 {published data only}
-
- Gaber AO, Egidi MF, Lo A, Gaber LW, Shokouh‐Amiri MH, Grewal HP, et al. Defining the optimal doses of sirolimus and tacrolimus in high‐risk cadaveric renal transplant recipients [abstract no: 541]. American Journal of Transplantation 2002;2(Suppl 3):274. [CENTRAL: CN‐00415690]
-
- Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus‐based calcineurin inhibitor‐sparing and calcineurin inhibitor‐free regimens in cadaveric renal transplantation. Transplantation 2004;77(8):1228‐35. [MEDLINE: ] - PubMed
-
- Lo A, Egidi MF, Gaber LW, Gaber AO. Observations on the use of sirolimus and tacrolimus in high‐risk renal transplant recipients. Transplantation Proceedings 2003;35(3 Suppl):105‐8S. [MEDLINE: ] - PubMed
-
- Lo A, Egidi MF, Gaber LW, Shokouh‐Amiri MH, Nazakatgoo N, Fisher JS, et al. Observations regarding the use of sirolimus and tacrolimus in high‐risk cadaveric renal transplantation. Clinical Transplantation 2004;18(1):53‐61. [MEDLINE: ] - PubMed
-
- Lo A, Egidi MF, Gross B, Amiri HS, Vera S, Nezakatgoo N, et al. The use of sirolimus and mycophenolate mofetil is associated with improved renal function, but similar metabolic profiles to sirolimus and tacrolimus [abstract no: 1408]. American Journal of Transplantation 2004;4(Suppl 8):544. [CENTRAL: CN‐00509328]
MacDonald‐302 2001 {published data only}
-
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. American Journal of Transplantation 2002;2(6):551‐9. [MEDLINE: ] - PubMed
-
- Campistol JM, Cole E, Gioud‐Paquet M, US European and Global Renal Transplant Study Group. Sirolimus (rapamune): effects on bone in renal transplant patients [abstract no: 681]. Transplantation 2000;69(8 Suppl):S289. [CENTRAL: CN‐00444659]
-
- Campistol JM, Legendre C, Castegneto M, Hartmann A, European US Global and Tri‐Continental Study Group. The effect of sirolimus on lipids on renal transplant recipients: results from clinical trials [abstract no: A4606]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):880A. [CENTRAL: CN‐00550752]
-
- Campistol JM, Legendre C, Hartmann A, Castagneto M, European US Global and Tri‐Continental Study Group. Lipid effects in renal allograft recipients receiving sirolimus‐based therapies [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00444660]
-
- Kahan BD, Jaffe JS. Triglyceride (TG) elevations in renal transplant recipients treated with sirolimus (rapamycin, RAPA) added to a cyclosporine (CSA)/prednisone (PRED) regimen [abstract no: 172]. Transplantation 1999;67(9):S585. [CENTRAL: CN‐00445975]
Machado 2001 {published data only}
-
- Machado PG, Felipe CR, Hanzawa NM, Park SI, Garcia R, Alfieri F, et al. An open‐label randomized trial of the safety and efficacy of sirolimus vs. azathioprine in living related renal allograft recipients receiving cyclosporine and prednisone combination. Clinical Transplantation 2004;18(1):28‐38. [MEDLINE: ] - PubMed
-
- Machado PG, Felipe CR, Park SI, Garcia R, Moreira S, Casarini D, et al. Preservation of graft function in low‐risk living kidney transplant recipients treated with a combination of sirolimus and cyclosporine. Brazilian Journal of Medical & Biological Research 2004;37(9):1303‐12. [MEDLINE: ] - PubMed
-
- Machado PG, Garcia C, Felipe CR, Garcia R, Franco M, Delcelo R, et al. A single‐center open label randomized trial of the safety and efficacy of the use of sirolimus versus azathioprine in one‐haplotype living related kidney transplant recipients‐preliminary results. Transplantation Proceedings 2001;33(1‐2):1074‐5. [MEDLINE: ] - PubMed
-
- Machado PP, Junior HT, Felipe CR, Garcia C. A single‐center open label randomized trial of the safety and efficacy of the use of rapamune versus azathioprine one haplotype living related kidney recipients ‐ preliminary results [abstract no: PO545]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00446521]
-
- Medina‐Pestana J, Felipe C, Franco M, Garcia R, Machado P, Tedesco H. A single‐center open label randomized trial of the safety and efficacy of the use of sirolimus versus azathioprine in one haplotype living related kidney recipients: 6 months analysis [abstract no: 1666]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00716080]
Martinez‐Mier 2006 {published data only}
-
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Avila‐Pardo SF, Zamudio‐Morales C. Living related kidney transplantation without calcineurin inhibitors: 3‐year results of a randomized prospective trial in a Mexican center [abstract no: 1098]. American Journal of Transplantation 2009;9(Suppl 2):500. [CENTRAL: CN‐01657972]
-
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [EMBASE: 2006628001] - PubMed
Morelon 2010 {published data only}
-
- Morelon E, Lefrancois N, Besson C, Prevautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: O‐259]. Transplant International 2009;22(Suppl 2):68‐9. - PubMed
-
- Morelon E, Lefrancois N, Besson C, Prevautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during T‐lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transplant Immunology 2010;23(1‐2):53‐8. [MEDLINE: ] - PubMed
-
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kollop‐Sarda MN, Brunet M, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: 622]. American Journal of Transplantation 2009;9(Suppl 2):371. [EMBASE: 70010495]
-
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kollop‐Sarda MN, Brunet M, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: P‐58]. Transplant International 2009;22(Suppl 2):109.
-
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kolopp‐Sarda MN, Brunet M, et al. Immune reconstitution in human renal allograft recipients treated by sirolimus or cyclosporin after thymoglobulin induction therapy [abstract no: 797]. Transplantation 2008;86(Suppl 2):278. [CENTRAL: CN‐00740582]
ORION 2011 {published data only}
-
- Campistol JM, Glyda M, Gurkan A, Flechner SM, Schulman S. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based regimens compared with calcineurin inhibitors (CNIs) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: P‐105]. Transplant International 2009;22(Suppl 2):121. [CENTRAL: CN‐01657955]
-
- Flechner S, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC)+ mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year safety results from the Orion Trial [abstract no: 444]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740477]
-
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the ORION study [abstract no: 1141]. American Journal of Transplantation 2007;7(Suppl 2):440.
-
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from the ORION study [abstract no: 52]. American Journal of Transplantation 2007;7(Suppl 2):160.
-
- Flechner S, Glyda M, Steinberg S, Harler MB, ORION Trial Investigators. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from The ORION Study [abstract no: P477]. Transplant International 2007;20(Suppl 2):209. [CENTRAL: CN‐01657180]
Paoletti 2012 {published data only}
-
- Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93(5):503‐8. [MEDLINE: ] - PubMed
-
- Paoletti E, Marsano L, Bellino D, Cassottana P, Rolla D, Maio G. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial [abstract no: 16]. American Journal of Transplantation 2012;12(Suppl 3):31. [EMBASE: 70745963]
Pascual 2010 {published data only}
-
- Castillo D, Pascual J, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. A phase II randomized pharmacokinetic (PK) study of tacrolimus (TAC)‐everolimus (EVL) combination for kidney transplantation (KT) [abstract no: 449]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740487]
-
- Pascual J, Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez A, et al. A phase II dose‐comparison randomized pharmacokinetic (PK) study on tacrolimus (TAC)‐everolimus (EVL) combination for kidney transplantation (KT) [abstract no: SP502]. NDT Plus 2008;1(Suppl 1):ii201.
-
- Pascual J, Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. Interaction between everolimus and tacrolimus in renal transplant recipients: a pharmacokinetic controlled trial. Transplantation 2010;89(8):994‐1000. [MEDLINE: ] - PubMed
Pescovitz 2007 {published data only}
-
- Pescovitz M, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety and efficacy of mycophenolate mofetil in combination with sirolimus vs cyclosporine in renal transplant patients [abstract no: 341]. American Journal of Transplantation 2004;4(Suppl 8):251. [CENTRAL: CN‐00509411] - PMC - PubMed
-
- Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. British Journal of Clinical Pharmacology 2007;64(6):758‐71. [MEDLINE: ] - PMC - PubMed
Qazi 2017 {published data only}
-
- Peddi V, Qazi Y, Shaffer D, Luan F, Shihab F, Tomlanovich S, et al. Effect of everolimus with low dose tacrolimus vs mycophenolate with standard tacrolimus regimen in African‐American de novo renal transplant recipients [abstract no: B955]. Transplantation 2014;98(Suppl 1):536. [EMBASE: 71545337]
-
- Qazi Y, Shaffer D, Kaplan B, Kim D, Luan F, Peddi V, et al. Efficacy and safety of everolimus with low‐dose tacrolimus in de novo renal transplant recipients: 12‐month randomized study [abstract no: 713]. Transplantation 2014;98(Suppl 1):80. [EMBASE: 71543789] - PubMed
-
- Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR, et al. Efficacy and safety of everolimus plus low‐dose tacrolimus versus mycophenolate mofetil plus standard‐dose tacrolimus in de novo renal transplant recipients: 12‐month data. American Journal of Transplantation 2017;17(5):1358‐69. [MEDLINE: ] - PubMed
-
- Shaffer D, Qazi Y, Kim D, Mulgaonkar S, Shihab F, Tomlanovich S, et al. Management of the wound complications in de novo renal transplant recipients: US92 12‐month randomized study [abstract no: B975]. Transplantation 2014;98(Suppl 1):542‐3. [EMBASE: 71545357]
-
- Shihab F, Qazi Y, Kaplan B, Kim D, Mulgaonkar S, Peddi V, et al. Everolimus‐facilitated tacrolimus minimization preserves renal function in de novo renal transplant recipients [abstract no: B962]. Transplantation 2014;98(Suppl 1):538‐9. [EMBASE: 71545344]
RECORD 2017 {published data only}
-
- Huh KH, Lee JG, Ha J, Oh CK, Ju MK, Kim CD, et al. De novo low‐dose sirolimus versus mycophenolate mofetil in combination with extended‐release tacrolimus in kidney transplant recipients: a multicentre, open‐label, randomized, controlled, non‐inferiority trial. Nephrology Dialysis Transplantation 2017;32(8):1415‐24. [MEDLINE: ] - PubMed
-
- Lee J, Huh K, Ha J, Oh CK, Ju M, Kim CD, et al. De novo low‐dose sirolimus versus mycophenolate mofetil in combination with extended‐release tacrolimus in kidney transplant recipients [abstract no: 72]. American Journal of Transplantation 2017;17(Suppl 3):231‐2. [EMBASE: 615704942] - PubMed
Riad 2007 {published data only}
-
- Riad H, Griffin C, Parrott N, Augustine T, Campbell B, Pararajasingam R, et al. Randomised prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard ciclosporin based triple therapy for rejection prophylaxis in renal transplantation: results at year 4 [abstract no: O39]. British Transplantation Society (BTS).13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
-
- Riad H, Tavakoli A, Hamer C, Augustine T, Parrott N, Nimako L, et al. A randomized prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard cyclosporin based triple therapy for rejection prophylaxis in renal transplantation [abstract no: P086]. Transplant International 2007;20(Suppl 2):116. [CENTRAL: CN‐00740555]
-
- Tavakoli A, Riad H, Hamer C, Augustine T, Parrott N, Nimako L, et al. A randomised prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard cyclosporin based triple therapy for rejection prophylaxis in renal transplantation [abstract no: O17]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
Rostaing 2001 {published data only}
-
- Rostaing L, Tran‐Van T, Cointault O, Lavayssiere L, Durand D, Ader JL. Assessment of renal function in de novo renal transplant patients receiving either sirolimus or everolimus in addition to ciclosporine A [abstract no: A4785]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):916A. [CENTRAL: CN‐00550538]
Russ 2003 {published data only}
-
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Comparison of reduced‐and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 21st Annual Scientific Meeting; 2003 Apr 9‐11; Canberra, Australia. 2003:64. [CENTRAL: CN‐00447521]
-
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. The safety and efficacy of reduced‐ and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results from Australia [abstract no: SA‐P0496]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):364A. [CENTRAL: CN‐00447522]
-
- Russ GR, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Reduced and standard target concentration tacrolimus with sirolimus in renal allograft recipients. Transplantation Proceedings 2003;35(3 Suppl):115‐7S. [MEDLINE: ] - PubMed
-
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract no: 1218]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351]
Sampaio 2008 {published data only}
-
- Park SI, Felipe CR, Pinheiro‐Machado PG, Garcia R, Fernandes FB, Casarini DE, et al. Tacrolimus pharmacokinetic drug interactions: effect of prednisone, mycophenolic acid or sirolimus. Fundamental & Clinical Pharmacology 2009;23(1):137‐45. [MEDLINE: ] - PubMed
-
- Sampaio EL, Pinheiro‐Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, et al. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus‐based immunosuppressive regimen. Clinical Transplantation 2008;22(2):141‐9. [MEDLINE: ] - PubMed
Schaefer 2006 {published data only}
-
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short‐term results under three different immunosuppressive regimens at one center. Transplantation Proceedings 2006;38(10):3466‐7. [MEDLINE: ] - PubMed
Shetty 2015 {published data only}
-
- Gallon L, Leventhal J, Maluf D, Sai B, Shetty A, Traitanon O, et al. Molecular profiles of renal allograft biopsies at 12 months post renal transplant in patients exposed to low dose CNI and everolimus vs. patients exposed to full dose CNI and MMF [abstract no: 69]. American Journal of Transplantation 2017;17(Suppl 3):230. [EMBASE: 615704855]
-
- Shetty A, Leventhal J, Traitanon O, Alvarado A, Mas V, Mathew J, et al. Prospective study of a steroid free, low dose tacrolimus and everolimus combination regimen in kidney transplant [abstract no: D133]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71954083]
-
- Shetty A, Opas T, Mathew J, Mas V, Leventhal J, Sustento‐Reodica N, et al. Impact of low dose tacrolimus with everolimus regimen on renal pathology and t‐regulatory cells in kidney transplant [abstract no: 38]. American Journal of Transplantation 2016;16(Suppl 3):218. [EMBASE: 611700367]
-
- Shetty A, Traitanon O, Ansari M, Mathew J, Leventhal J, Mas V, et al. Prospective randomized study of a steroid free, low dose tacrolimus with everolimus regimen in kidney transplant [abstract no: B110]. American Journal of Transplantation 2016;16(Suppl 3):535‐6. [EMBASE: 611700126]
Souza 2017 {published data only}
-
- Souza P, Ventura C, Agena F, Barreto N, Reusing JO, Onusic V, et al. Impact of everolimus added to tacrolimus/ mycophenolate/prednisone on CMV disease and acute rejection in sensitized kidney transplant recipients. A single center, randomized and controlled, pilot study [abstract no: P341]. Transplant International 2017;30(Suppl 2):482. [EMBASE: 618770379]
Spagnoletti 2017 {published data only}
-
- Spagnoletti G, Gesualdo L, Pisani F, Gruttadauria S, Caputo F, Frasca G, et al. Multicenter, randomized, open label, prospective clinical study to compare the efficacy and safety of steroid withdrawal on day 5 in a combination of tacrolimus plus everolimus vs tacrolimus plus MMF [abstract no: OS163]. Transplant International 2017;30(Suppl 2):64. [EMBASE: 618769692]
Stallone 2004 {published data only}
-
- Stallone G, Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1‐year graft function. Journal of the American Society of Nephrology 2004;15(1):228‐33. [MEDLINE: ] - PubMed
-
- Stallone G, Infante B, Schena A, Paolo S, Gesualdo L, Ditonno P, et al. Sirolimus prolongs delayed graft function in recipients of sub‐optimal cadaveric kidney donors [abstract no: 1601]. American Journal of Transplantation 2003;3(Suppl 5):563. [CENTRAL: CN‐00447829]
Stegall 2003 {published data only}
-
- Dean PG, Grande JP, Sethi S, Park WD, Griffin MD, Cosio FG, et al. Kidney transplant histology after one year of continuous therapy with sirolimus compared with tacrolimus. Transplantation 2008;85(8):1212‐5. [MEDLINE: ] - PubMed
-
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effect of immunosuppression on renal function and graft histology: a comparison of tacrolimus and sirolimus [abstract no: 379]. American Journal of Transplantation 2005;5(Suppl 11):252. [CENTRAL: CN‐00725015]
-
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effects of calcineurin inhibitor avoidance on renal function and graft histology after kidney transplantation: a prospective, randomized comparison of tacrolimus and sirolimus [abstract no: O228]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509154]
-
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus [abstract no: 259]. American Journal of Transplantation 2004;4(Suppl 8):229. [CENTRAL: CN‐01912356] - PubMed
-
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound‐healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77(10):1555‐61. [MEDLINE: ] - PubMed
SYMPHONY 2007 {published data only}
-
- Bagul A, Nicholson M, Chavez R, Grinyo J, Frei U, Vanrentergehm Y, et al. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY study [abstract no: P23]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007. [CENTRAL: CN‐01657142]
-
- Chavez R, Nicholson M, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, et al. SYMPHONY ‐ Comparing efficacy of standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation. Sub analysis of GFR in cases that completed one year within an intended regime [abstract no: O06]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
-
- Claes K, Meier‐Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrology Dialysis Transplantation 2012;27(2):850‐7. [MEDLINE: ] - PubMed
-
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981]
-
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐01912358]
Takahashi 2013a {published data only}
-
- Goto N, Watarai Y, Narumi S, Yamamoto T, Tsujita M, Hiramitsu T, et al. Lower incidence of cytomegalovirus infection with everolimus based immunosuppression versus mycophenolate in de novo renal transplants with 5 years follow‐up [abstract no: D2449]. Transplantation 2014;98(Suppl 1):621. [EMBASE: 71545639]
-
- Hiramitsu T, Okada M, Futamura K, Yamamoto T, Tsujita M, Goto N, et al. 5‐year follow‐up of a randomized clinical study comparing everolimus plus reduced‐dose cyclosporine with mycophenolate mofetil plus standard‐dose cyclosporine in de novo kidney transplantation: Retrospective single center assessment. International Immunopharmacology 2016;39:192‐8. [MEDLINE: ] - PubMed
-
- Nakagawa K, Kenmochi T, Watarai Y, Kamisawa O. Efficacy and safety of everolimus plus reduced‐dose cyclosporine in recipients of living‐related and unrelated kidney donors: A 24‐month sub‐analysis from the A1202 study [abstract no: P314]. Transplant International 2017;30(Suppl 2):474. [EMBASE: 618769145]
-
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Okada M, Tsujita M, et al. Long‐term efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 9 years follow‐up [abstract no: C86]. American Journal of Transplantation 2018;18(Suppl 4):797. [EMBASE: 622280454]
-
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Okada M, et al. Everolimus‐based Immunosuppression Possibly Suppresses Mean Fluorescence Intensity Values of De Novo Donor‐specific Antibodies After Primary Kidney Transplantation. Transplantation Proceedings 2019;51(5):1378‐81. [MEDLINE: ] - PubMed
Tedesco‐Silva 2003 {published data only}
-
- Ferreira A, Medina‐Pestana J, Pinheiro‐Machado P, Felipe C, Motegi S, Hosaka B, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients: 12‐month results [abstract no: P321]. Transplantation 2004;78(2 Suppl):307. [CENTRAL: CN‐00509189]
-
- Ferreira AN, Machado PG, Felipe CR, Motegi SA, Hosaka BH, Tanaka MK, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients: 12‐month results [abstract no: 458]. American Journal of Transplantation 2004;4(Suppl 8):284. [CENTRAL: CN‐01912454]
-
- Ferreira AN, Machado PG, Felipe CR, Motegi SA, Hosaka BH, Tanaka MK, et al. Concentration‐controlled use of sirolimus associated with reduced exposure of cyclosporine in black recipients of primarily living renal allograft donors: 12‐month results. Clinical Transplantation 2005;19(5):607‐15. [MEDLINE: ] - PubMed
-
- Machado PG, Felipe CR, Park SI, Garcia R, Franco M, Alfieri F, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients [abstract no: 777]. American Journal of Transplantation 2003;3(Suppl 5):351. [CENTRAL: CN‐00446520]
-
- Machado PG, Hosaka BH, Felipe CR, Garcia R, Franco M, Alfieri F, et al. A concentration‐controlled study of the use of sirolimus (SRL)‐cyclosporine (CSA) combination in black patients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416199]
Tedesco‐Silva 2010 {published data only}
-
- Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 43]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 June 29‐Jul 1; Canberra (ACT). 2011:64. [CENTRAL: CN‐01657183]
-
- Carmellini M, Garcia V, Wang Z, Vergara M, Escrig C, Russ G. Everolimus de novo with reduced‐exposure cyclosporine in renal transplant recipients at high risk of efficacy failure: results of a post‐HOC analysis [abstract no: BO234]. Transplant International 2015;28(Suppl 4):209. [EMBASE: 72111829]
-
- Carmellini M, Garcia V, Wang Z, Vergara M, Russ G. Efficacy of everolimus with reduced‐exposure cyclosporine in de novo kidney transplant patients at increased risk for efficacy events: analysis of a randomized trial. Journal of Nephrology 2015;28(5):633‐9. [MEDLINE: ] - PubMed
-
- Carmellini M, Garcia V, Wong Z, Vergara M, Escrig C, Russ G. Treatment with everolimus and reduced‐exposure cyclosporine is efficacious in de novo renal transplant recipients at increased risk for efficacy failure: post‐hoc analysis from the A2309 study [abstract no: D125]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71954404]
-
- Chadban S, Pilmore H, Russ G, John K, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: Long‐term follow‐up of Australia and New Zealand kidney transplant recipients in the A2309 randomised controlled trial [abstract no: 450.9]. Transplantation 2016;100(7 Suppl 1):S250. [EMBASE: 613005419]
Tedesco‐Silva 2015 {published data only}
-
- Basso G, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes‐Freitas T, et al. Kinetics of cytomegalovirus load in kidney transplant recipients receiving everolimus or mycophenolate sodium and no pharmacological prophylaxis [abstract no: C274]. American Journal of Transplantation 2016;16(Suppl 3):688. [EMBASE: 611699076]
-
- Basso G, Felipe CR, Cristelli MP, Mansur Silviano J, Viana L, Ferreira Brigido AN, et al. The effect of anti‐thymocyte globulin and everolimus on the kinetics of cytomegalovirus viral load in seropositive kidney transplant recipients without prophylaxis. Transplant Infectious Disease 2018;20(4):e12919. [MEDLINE: ] - PubMed
-
- Brigido A, Tedesco H, Felipe C, Cristelli M, Franco M, Medina‐Pestana J. Efficacy and renal function in kidney transplant recipients receiving tacrolimus (TAC)‐based immunosuppressive regimens in combination with everolimus (EVR) versus mycophenolate (MPA) [abstract no: 64]. American Journal of Transplantation 2016;16(Suppl 3):227. [EMBASE: 611699395]
-
- Cristelli M, Felipe C, Oliveira N, Gusukuma L, Ferreira A, Sandes‐Freitas T, et al. De novo everolimus (EVR) versus mycophenolate (MPA) in kidney transplant recipients receiving tacrolimus (TAC) [abstract no: 2908]. Transplantation 2014;98(Suppl 1):141. [EMBASE: 71543976]
TRANSFORM 2018 {published data only}
-
- Berger S, Sommerer C, Bakr M, Soo KM, Danguilan R, Fijter J, et al. TRANSFORM study: Impact of donor type on 12‐month outcomes of everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in de novo kidney transplant patients [abstract no: LOS011]. Transplant International 2017;30(Suppl 2):162‐3. [EMBASE: 618769281]
-
- Berger SP, Sommerer C, Witzke O, Tedesco H, Chadban S, Mulgaonkar S, et al. Two‐year outcomes in de novo renal transplant recipients receiving everolimus‐facilitated calcineurin inhibitor reduction regimen from TRANSFORM study. American Journal of Transplantation 2019 Jun 1 [epub ahead of print]. [PUBMED: 31152476] - PubMed
-
- Buchler M, Sommerer C, Mulgaonkar S, Garcia V, Massari P, Kuypers D, et al. The TRANSFORM study: lower viral infections with everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in de novo kidney transplant patients at month 12 [abstract no: O40]. Transplant International 2018;31(Suppl 1):14. [EMBASE: 619997597]
-
- Chadban S, Hughes P, Campbell S, Irish A, Lim W, O'Connell P, et al. TRANSFORM trial design: a randomized, multicentre, open‐label study of everolimus with reduced calcineurin inhibitors in over 20000 de novo renal transplant recipients [abstract no: 48]. Transplantation Society of Australia & New Zealand (TSANZ). 32nd Annual Scientific Meeting; 2014 Jun 11‐13; Canberra (ACT). 2014:61. [CENTRAL: CN‐01657286]
-
- Chadban S, Mulgaonkar S, Pascual J, Tedesco H, Berger S, Qazi Y, et al. Everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplant recipients: efficacy and safety outcomes from the TRANSFORM study [abstract no: 597]. American Journal of Transplantation 2018;18(Suppl 4):475. [EMBASE: 622280975]
van Gurp 2010 {published data only}
-
- Gurp E, Bustamante J, Franco A, Rostaing L, Becker T, Rondeau E, et al. Comparable renal function at 6 months with tacrolimus combined with fixed‐dose sirolimus or MMF: results of a randomized multicenter trial in renal transplantation. Journal of Transplantation 2010;2010. [MEDLINE: ] - PMC - PubMed
van Hooff 2003 {published data only}
-
- Wlodarczyk Z, Squifflet JP, Hooff JP, Vanrentergheim Y, Paczek L. Tacrolimus in combination with rapamycin: results of a multicentre, randomised, dose‐finding study [abstract no: 1314]. American Journal of Transplantation 2002;2(Suppl 3):469. [CENTRAL: CN‐00416943]
-
- Wlodarczyk Z, Hooff JP, Vanrenterghem Y, Squifflet JP, Paczek L. Tacrolimus in combination with various dosages of rapamycin in renal recipients: safety and efficacy of the first 6‐month multicenter randomised trial [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00725007]
-
- Hooff JP, Squifflet JP, Wlodarczyk Z, Vanrenterghem Y, Paczek L. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal‐transplant recipients. Transplantation 2003;75(12):1934‐9. [MEDLINE: ] - PubMed
Velosa‐212 2001 {published data only}
-
- Campistol JM, Alveranga D, Hricik DE, Velosa J, Grinyo JM, Mourad G, et al. Sirolimus‐treated renal transplant recipients experience improved renal function with cyclosporine elimination: one‐year results from a phase II trial [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00487674]
-
- Gonwa T, Alveranga D, Ancona G, Brinker K, Cambi V, Campistol J, et al. Sirolimus (rapamune) permits early elimination of cyclosporine in recipients of cadaveric renal allografts [abstract no: 958]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00445514]
-
- Gonwa TA. Sirolimus (SRL) treated patients demonstrate improved renal function after withdrawal of cyclosporine (CSA) [abstract no: 4658]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):891A. [CENTRAL: CN‐00550647]
-
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] - PubMed
-
- Hricik DE, Rapamune Renal Function Study Group. Sirolimus improves renal function without increasing acute rejection after cyclosporine elimination in renal transplant patients [abstract no: 0430]. XVIII International Congress of the Transplantation Society; 2000 Aug 27 ‐ Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00583304]
Vitko‐201 2001 {published and unpublished data}
-
- Boger R, Dantal J, Margreiter R, Viljoen H, Vitko S, Weimar W, et al. CERTICAN (TM) (RAD; Everolimus), the proliferation signal inhibitor, is complementary with Neoral in preventing acute allograft rejection [abstract no: 1626]. 2001 A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487752]
-
- Dantal J, Boger R, RAD 201 Study Group, Margreiter R, Viljoen H, Vitko S, et al. Incidence of cytomegalovirus and other viral infections is significantly less in patients receiving Certican(® (RAD; Everolimus) versus MMF while maintaining good efficacy [abstract no: 1624]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00583727]
-
- Dantal J, Vitko S, Margreiter R, Weimar W, Viljoen H, Boger R, et al. Incidence of cytomegalovirus and other viral infections is significantly less in patients receiving certican (RAD; everolimus) versus MMF while maintaining good efficacy [abstract no: 404]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00527106]
-
- Dantal J, Vitko S, Margreiter R, Weimar W, Viljoen H, Somberg K. Everolimus (certican (TM), RAD) is associated with a reduced incidence of CMV infection following renal transplantation [abstract no: 963]. American Journal of Transplantation 2002;2(Suppl 3):380. [CENTRAL: CN‐00415492]
-
- Holmes M, Chilcott J, Walters S, Whitby S, Akehurst R. Economic evaluation of everolimus versus mycophenolate mofetil in combination with cyclosporine and prednisolone in de novo renal transplant recipients. Transplant International 2004;17(4):182‐7. [MEDLINE: ] - PubMed
Vitko‐TERRA 2004 {published and unpublished data}
-
- Margreiter R, Czajkowski Z, Vitko S, Wlodarczyk W, Salmela K, TERRA Study Group. Renal graft function and proteinuria in patients receiving tacrolimus/sirolimus combination therapy [abstract no: P‐62]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550627]
-
- Salmela K, Vitko S, Wlodarczyk Z, Czajkowski Z, Margreiter R, TERRA Study Group. Tacrolimus with MMF or two different doses of sirolimus in kidney transplantation: a large randomised multicentre study [abstract no: 1630]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00583243]
-
- Vitko S, Wlodarczyk Z, Kyllonen L, Czajkowski Z, Margreiter R, Backman L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. American Journal of Transplantation 2006;6(3):531‐8. [MEDLINE: ] - PubMed
-
- Vitko S, Wlodarczyk Z, Salmela K, Czajkowski Z, Margreiter R. Tacrolimus in combination with two different sirolimus doses versus a tacrolimus/MMF‐based regimen: a large, randomised clinical study in renal transplantation [abstract no: 0286]. Transplantation 2004;78(2 Suppl):112‐3. [CENTRAL: CN‐00509547]
-
- Wlodarczyk Z, Vitko S, Salmela K, Czajkowski Z, Margreiter R, TERRA Study Group. Lipid metabolism in renal transplant patients receiving tacrolimus/sirolimus combination therapy. Transplantation Proceedings 2005;37(4):1871‐3. [MEDLINE: ] - PubMed
References to studies excluded from this review
ADHERE 2017 {published data only}
-
- Rummo OO, Carmellini M, Rostaing L, Oberbauer R, Christiaans MH, Mousson C, et al. ADHERE: randomized controlled trial comparing renal function in de novo kidney transplant recipients receiving prolonged‐release tacrolimus plus mycophenolate mofetil or sirolimus. Transplant International 2017;30(1):83‐95. [MEDLINE: ] - PubMed
Barsoum 2007 {published data only}
-
- Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo Kidney Center protocol for rapamycin‐based sequential immunosuppression in kidney transplant recipients: 2‐year outcomes. Experimental & Clinical Transplantation 2007;5(2):649‐57. [MEDLINE: ] - PubMed
CALLISTO 2009 {published data only}
-
- Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Blanc AS, et al. Immediate versus delayed everolimus treatment in de novo renal transplant recipients at risk of delayed graft function: 1‐year results of the CALLISTO Study [abstract no: P‐99]. Transplant International 2009;22(Suppl 2):119.
-
- Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Incidence of delayed graft function and wound healing complications after deceased‐donor kidney transplantation is not affected by de novo everolimus. Transplantation 2009;88(1):69‐76. [MEDLINE: ] - PubMed
-
- Cassuto E, Kamar N, Berthoux F, Moal M, Legendre C, Blanc A, et al. Renal function and wound healing in kidney transplant recipients at risk of delayed graft function receiving everolimus [abstract no: 1089]. American Journal of Transplantation 2009;9(Suppl 2):498. [CENTRAL: CN‐00776097]
-
- Dantal J, Berthoux F, Moal M, Rostaing L, Legendre C, Blanc A, et al. Immediate vs delayed everolimus treatment in de novo renal transplant patients at risk of delayed graft function: results of a 12‐month randomized, multicentre trial [abstract no: 239]. American Journal of Transplantation 2009;9(Suppl 2):259. [CENTRAL: CN‐00775108]
-
- Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Blanc AS, et al. Immediate versus delayed everolimus: comparable renal function and wound healing complications in kidney transplant recipients at risk of delayed graft function [abstract no: O‐301]. Transplant International 2009;22(Suppl 2):79.
Carmellini 2010 {published data only}
-
- Carmellini M, Bernini M, Ruggieri G, Collini A. Conversion of stable kidney transplant recipients from a twice‐daily everolimus regimen to a once‐daily everolimus regimen [abstract no: 1109]. American Journal of Transplantation 2009;9(Suppl 2):503. [CENTRAL: CN‐00774238] - PubMed
-
- Carmellini M, Collini A, Ruggieri G, Bernini M. Conversion of stable kidney transplant recipients from a twice‐daily to once‐daily everolimus regimen. Transplantation Proceedings 2010;42(4):1312‐3. [MEDLINE: ] - PubMed
CENTRAL 2012 {published data only}
-
- Mjornstedt L, Schwartz Sorensen S, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transplant International 2015;28(1):42‐51. [MEDLINE: ] - PubMed
-
- Mjornstedt L, Sorensen SS, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. American Journal of Transplantation 2012;12(10):2744‐53. [MEDLINE: ] - PubMed
-
- Murbraech K, Holdaas H, Massey R, Undset LH, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients: an echocardiographic substudy of the randomized controlled CENTRAL trial. Transplantation 2014;97(2):184‐8. [MEDLINE: ] - PubMed
-
- Murbraech K, Massey R, Undset LH, Midtvedt K, Holdaas H, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients ‐ a three‐yr serial echocardiographic substudy of the randomized controlled CENTRAL trial. Clinical Transplantation 2015;29(8):678‐84. [MEDLINE: ] - PubMed
CERTITEM 2015 {published data only}
-
- Hertig A, Kamar N, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: 717]. Transplantation 2014;98(Suppl 1):81. [EMBASE: 71543793]
-
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: O5]. Transplant International 2013;26(Suppl 3):2. [EMBASE: 71356080]
-
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: the CERTITEM trial [abstract no: P081]. Transplant International 2013;26(Suppl 2):201. [EMBASE: 71359712]
-
- Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Fibrosis progression according to epithelial‐mesenchymal transition profile: a randomized trial of everolimus versus CsA. American Journal of Transplantation 2015;15(5):1303‐12. [MEDLINE: ] - PubMed
-
- Snanoudj R, Suberbielle C, Kamar N, Cassuto E, Caillard S, Taupin JL, et al. Epitope load is predictive of de novo donor specific antibodies occurrence in renal transplant recipients after conversion from cyclosporine to everolimus [abstract no: 279]. American Journal of Transplantation 2016;16(Suppl 3):301‐2. [EMBASE: 611699953]
Citterio 2004 {published data only}
-
- Citterio F, Rossi E, Tondolo V, Romagnoli J, Castagneto M. Better cardiovascular risk profile with tacrolimus based immunosuppression [abstract no: P‐60]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550362]
Cruzado 2016 {published data only}
-
- Cruzado JM, Pascual J, Sanchez‐Fructuoso A, Seron D, Diaz JM, Rengel M, et al. Controlled randomized study comparing the cardiovascular profile of everolimus with tacrolimus in renal transplantation. Transplant International 2016;29(12):1317‐28. [MEDLINE: ] - PubMed
-
- Morales J, Pascual J, Sanchez‐Fructuoso A, Seron D, Diaz J, Oppenheimer F, et al. Effect of everolimus versus tacrolimus on left ventricular hypertrophy in renal transplant patients on maintenance [abstract no: A176]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953375]
EVIDENCE 2014 {published data only}
-
- Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve‐month outcomes from EVIDENCE trial (everolimus once‐a‐day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients [abstract no: O193]. Transplant International 2013;26(Suppl 2):100. [EMBASE: 71359334]
-
- Ponticelli C, Carmellini M, Tisone G, Sandrini S, Segoloni G, Rigotti P, et al. A randomized trial of everolimus and low‐dose cyclosporine in renal transplantation: with or without steroids?. Transplantation Proceedings 2014;46(10):3375‐82. [MEDLINE: ] - PubMed
Fior 2015 {published data only}
-
- Fior F, Momo R, Nacchia F, Sagliocca A, Pessolano G, Boschiero L. Everolimus based/calcineurin inhibitors free protocol in marginal donor kidney transplantation: Results at five years of a cohort study [abstract no: P336]. Transplant International 2015;28(Suppl 4):504. [EMBASE: 72112316]
Libetta 2007 {published data only}
-
- Libetta C, Portalupia V, Sepe V, Cosmai L, Bellotti N, Rossi N, et al. Effects of sirolimus or cyclosporine‐based immunosuppression on in vitro allostimulation of CD4+ [abstract no: FP542]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi203. [CENTRAL: CN‐00724875]
-
- Libetta C, Sepe V, Zucchi M, Portalupi V, Meloni F, Rampino T, et al. The effect of sirolimus‐ or cyclosporine‐based immunosuppression effects on T‐cell subsets in vivo. Kidney International 2007;72(1):114‐20. [MEDLINE: ] - PubMed
Libetta 2015 {published data only}
-
- Libetta C, Esposito P, Gregorini M, Margiotta E, Martinelli C, Borettaz I, et al. Sirolimus vs cyclosporine after induction with basiliximab does not promote regulatory T cell expansion in de novo kidney transplantation: results from a single‐center randomized trial. Transplant Immunology 2015;33(2):117‐24. [MEDLINE: ] - PubMed
Mathew 2006 {published data only}
-
- Hariharan S, Zimmerman JJ, Rapamune Study Group. A comparative study of the pharmacokinetic profiles of sirolimus oral solution and tablets in renal allograft patients [abstract no:157]. Transplantation 2000;69(8 Suppl):S154.
-
- Hariharan S, Rapamune US Study Group. Equivalent safety and efficacy of sirolimus oral solution and tablet formulations in primary renal allograft recipients [abstract no: A3627]. Journal of the American Society of Nephrology 2000;11(Sept):690A. [CENTRAL: CN‐00550609]
-
- Mathew TH, Buren C, Kahan BD, Butt K, Hariharan S, Zimmerman JJ, et al. A comparative study of sirolimus tablet versus oral solution for prophylaxis of acute renal allograft rejection. Journal of Clinical Pharmacology 2006;46(1):76‐87. [MEDLINE: ] - PubMed
-
- Buren CT, Rapamune US Study Group. Sirolimus oral solution and tablet formulations demonstrate equivalent safety and efficacy in renal allograft recipients [abstract no:P0533]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome (Italy). 2000.
-
- Buren CT, Rapamune Study Group. Sirolimus oral solution and tablets demonstrate equivalent safety and efficacy in renal allograft [abstract no: 157]. Transplantation 2000;69(8 Suppl):S153‐4. [CENTRAL: CN‐00448115]
Nafar 2012 {published data only}
-
- Nafar M, Alipour B, Ahmadpoor P, Pour‐Reza‐Gholi F, Samadian F, Samavat S, et al. Sirolimus versus calcineurin inhibitor‐based immunosuppressive therapy in kidney transplantation: a 4‐year follow‐up. Iranian Journal of Kidney Diseases 2012;6(4):300‐6. [MEDLINE: ] - PubMed
NCT00005113 {published data only}
-
- NCT00005113. A study to compare treatment with sirolimus versus standard treatment in patients who have received a kidney transplant. www.clinicaltrials.gov/ct2/show/NCT00005113 (first received 14 April 2000).
NCT00965094 {published data only}
-
- NCT00965094. Efficacy and safety of everolimus+EC‐MPS after early CNI elimination vs EC‐MPS +tacrolimus in renal transplant recipients. www.clinicaltrials.gov/ct2/show/NCT00965094 (first received 24 August 2009).
nEVEROLD 2017 {published data only}
-
- David‐Neto E, Agena F, Ramos F, Triboni A, Altona M, Coelho V, et al. Everolimus/low tacrolimus (TAC) compared to MPA/regular TAC for renal transplantation in the elderly recipient‐preliminary analysis of the NEVEROLD trial [abstract no: 288]. American Journal of Transplantation 2016;16(Suppl 3):305. [EMBASE: 611700340]
-
- David‐Neto E, Agena F, Ramos F, Triboni AH, Altona M, Coelho V, et al. Everolimus/low tacrolimus (TAC) compared to MPA/regular TAC for renal transplantation in the elderly recipient‐preliminary analysis of the nEverOld trial [abstract no: P1481]. Transplantation 2016;100(7 Suppl 1):S694. [EMBASE: 613005024]
-
- David‐Neto E, Agena F, Ramos F, Triboni AH, Romano P, Almeida Rezende Ebner P, et al. Longitudinal pharmacokinetics of everolimus when combined with low‐level of tacrolimus in elderly renal transplant recipients. Transplantation 2017;101(9):2133‐8. [MEDLINE: ] - PubMed
-
- David‐Neto E, Romano P, Kamada Triboni AH, Ramos F, Agena F, Almeida Rezende Ebner P, et al. Longitudinal pharmacokinetics of tacrolimus in elderly compared with younger recipients in the first 6 months after renal transplantation. Transplantation 2017;101(6):1365‐72. [MEDLINE: ] - PubMed
-
- Romano P, Agena F, Almeida Rezende EP, Massakazu SN, Kamada Triboni AH, Ramos F, et al. Longitudinal pharmacokinetics of mycophenolic acid in elderly renal transplant recipients compared to a younger control group: data from the nEverOld trial. European Journal of Drug Metabolism & Pharmacokinetics 2019;44(2):189‐99. [MEDLINE: ] - PubMed
NEVERWOUND 2014 {published data only}
-
- Carmellini M, Boschiero L, Rigotti P, Sandrini S, Frasca GM, Minetti E, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in kidney transplant recipients: 12‐months results from NEVERWOUND study [abstract no: BOS299]. Transplant International 2017;30(Suppl 2):252. [EMBASE: 618770335]
-
- Carmellini M, Ruggieri G, Sforza D, Veroux M, Secchi A, Todeschini P, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in renal transplant patients: Preliminary results from NEVERWOUND study [abstract no: D2430]. Transplantation 2014;98(Suppl 1):615. [EMBASE: 71545620]
-
- Carmellini M, Todeschini P, Secchi A, Sandrini S, Minetti E, Furian L, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in kidney transplant recipients: 3‐months results from NEVERWOUND study [abstract no: 67]. American Journal of Transplantation 2016;16(Suppl 3):228. [EMBASE: 611699536]
Novoa 2011 {published data only}
-
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus CSA elimination compared with CSA reduction [abstract no: P‐359]. Transplant International 2009;22(Suppl 2):183. [CENTRAL: CN‐01657137]
-
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation [abstract no: 1636]. American Journal of Transplantation 2010;10(Suppl 4):503. [EMBASE: 70465011]
-
- Novoa PA, Grinyo JM, Ramos FJP, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplantation Proceedings 2011;43(9):3331‐9. [MEDLINE: ] - PubMed
Oh 2012 {published data only}
-
- Oh CK, Ha JW, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus (Certican) with low dose of cyclosporine in de novo kidney recipients after 1 month of transplantation (preliminary results). Journal of the Korean Society for Transplantation 2012;26(2):83‐91. [CENTRAL: CN‐01046131]
-
- Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced‐exposure cyclosporine a in de novo kidney recipients. Transplantation 2015;99(1):180‐6. [MEDLINE: ] - PubMed
Pretagostini 2016 {published data only}
-
- Pretagostini R, Poli L, Pettorini L, Lai Q, Garofalo M, Melandro F, et al. Delayed introduction of everolimus in de novo renal transplanted patients: a single‐center experience. Transplantation Proceedings 2016;48(2):326‐8. [MEDLINE: ] - PubMed
Rivelli 2014 {published data only}
-
- Rivelli RF, Goncalves RT, Leite M Jr, Santos MA, Delgado AG, Cardoso LR, et al. Early withdrawal of calcineurin inhibitor from a sirolimus‐based immunosuppression stabilises fibrosis and the TGF‐beta signalling pathway in kidney transplant. Nephrology 2014;20(3):168‐76. [MEDLINE: ] - PubMed
SOCRATES 2014 {published data only}
-
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103. [CENTRAL: CN‐01912372]
Tamashiro 2017 {published data only}
-
- Tamashiro EY, Felipe CR, Genvigir FD, Rodrigues AC, Campos AB, Hirata RD, et al. Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metabolism & Personalized Therapy 2017;32(2):89‐95. [MEDLINE: ] - PubMed
van Gelder 2003 {published data only}
-
- Gelder T, ter Meulen CG, Hene R, Weimar W, Hoitsma A. Oral ulcers in kidney transplant recipients treated with sirolimus and mycophenolate mofetil. Transplantation 2003;75(6):788‐91. [MEDLINE: ] - PubMed
Wojciechowski 2017 {published data only}
-
- Webber A, Wojciechowski D, Leung C, Chandran S, Hirose R, Vincenti F. Pharmacodynamic monitoring of NFAT‐regulated gene expression and P70S6 kinase activity in kidney transplant patients with BKV infection [abstract]. Transplantation 2014;98(Suppl 1):880. [EMBASE: 71546539]
-
- Wojciechowski D, Chandran S, Webber A, Hirose R, Vincenti F. Mycophenolate mofetil withdrawal with conversion to everolimus to treat BK virus infection in kidney transplant recipients. Transplantation Proceedings 2017;49(8):1773‐8. [MEDLINE: ] - PubMed
-
- Wojciechowski D, Webber A, Chandran S, Hirose R, Vincenti F. Everolimus conversion to treat BK virus infection in renal transplant recipients: Interim analysis of a pilot study [abstract]. Transplantation 2014;98(Suppl 1):558‐9. [EMBASE: 71545412]
-
- Wojciechowski D, Webber A, Chandran S, Vincenti F. Everolimus conversion to treat BK virus infection in kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953446] - PubMed
Wyrley‐Birch 2009 {published data only}
-
- Wyrley‐Birch H, Kanwar A, Dakshinamoorty V, Navarro A, Reddy M, Asher J, et al. A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non heart beating donor kidney transplantation [abstract no: O41]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
-
- Wyrley‐Birch H, Kanwar A, Vijayanand D, Ray C, Moir J, Navarro A, et al. Early results from a trial comparing sirolimus with tacrolimus as primary immunosuppression for recipients of non heart beating donor (NHBD) kidneys after anti‐IL2 monoclonal antibody [abstract no: P‐580]. Transplant International 2009;22(Suppl 2):239.
References to studies awaiting assessment
Ferreira 2019 {published data only}
-
- Ferreira AN, Felipe CR, Cristelli M, Viana L, Mansur J, Paula M, et al. Prospective randomized study comparing everolimus and mycophenolate sodium in de novo kidney transplant recipients from expanded criteria deceased donor. Transplant International 2019;32(11):1127‐43. [MEDLINE: ] - PubMed
-
- Tedesco H, Nicolau FA, Cristelli M, Oliveira NI, Rosso FC, Pestana JO. Everolimus versus mycophenolate for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin and tacrolimus [abstract no: BO5]. Transplant International 2015;28(Suppl 4):135. [EMBASE: 72111605]
-
- Tedesco‐Silva H, Felipe C, Brigido A, Bessa A, Paula M, Ruppel P, et al. Everolimus (EVR) versus mycophenolate sodium (MPS) for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin (r‐ATG) and tacrolimus (TAC) [abstract no: 222]. American Journal of Transplantation 2016;16(Suppl 3):281. [EMBASE: 611699738]
-
- Tedesco‐Silva H, Felipe C, Ferreira A, Cristelli M, Bessa A, Ueno P, et al. Everolimus versus mycophenolate for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin and tacrolimus [abstract no: 294]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953354]
-
- Tedesco‐Silva H, Felipe CR, Cristelli MP, Viana LA, Ferreira AN, Bessa AB, et al. Everolimus (EVR) versus mycophenolate sodium (MPS) for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin (r‐ATG) and tacrolimus (TAC) [abstract no: 620.5]. Transplantation 2016;100(7 Suppl 1):S398. [EMBASE: 613004941]
Traitanon 2019 {published data only}
-
- Traitanon O, Mathew JM, Shetty A, Bontha SV, Maluf DG, Kassis Y, et al. Mechanistic analyses in kidney transplant recipients prospectively randomized to two steroid free regimen‐Low dose Tacrolimus with Everolimus versus standard dose Tacrolimus with Mycophenolate Mofetil. PLoS ONE 2019;14(5):e0216300. [MEDLINE: ] - PMC - PubMed
References to ongoing studies
EVER TWIST 2013 {published data only}
-
- Libetta C, Margiotta E, Borettaz I, Canevari M, Martinelli C, Lainu E, et al. Everolimus and low dose of tacrolimus combined with thymoglobulin induction induces regulatory T cells expansion in de novo kidney transplant recipients: Preliminary data of controlled randomized study (EVER TWIST) [abstract no: SP646]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i277. [EMBASE: 71075839]
NCT02077556 {published data only}
-
- Tsai MK. Effect of everolimus on the pharmacokinetics of tacrolimus in renal transplant patients. www.clinicaltrials.gov/ct2/show/NCT02077556 (first received 2 March 2014).
NCT03468478 {published data only}
-
- Tedesco Silva H Jr. Comparison of the efficacy and safety of sirolimus versus everolimus versus mycophenolate in kidney transplantation (SEM). www.clinicaltrials.gov/ct2/show/NCT03468478 (first received 9 March 2018).
Additional references
ANZDATA 2017
-
- ANZDATA Registry. 40th Report, Chapter 7: Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2018. www.anzdata.org.au/report/anzdata‐40th‐annual‐report‐2017/ (accessed 24 July 2019).
Dumont 2001
-
- Dumont FJ. Everolimus Novartis. Current Opinion in Investigational Drugs 2001;2(9):1220‐34. [MEDLINE: ] - PubMed
Egger 1997
Gondos 2013
-
- Gondos A, Dohler B, Brenner H, Opelz G. Kidney graft survival in Europe and the United States: strikingly different long‐term outcomes. Transplantation 2013;95(2):267‐74. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] - PubMed
Hernandez 2011
-
- Hernandez D, Martinez D, Gutierrez E, Lopes V, Gutierrez C, Garcia P, et al. Clinical evidence on the use of anti‐mTOR drugs in renal transplantation. Nefrologia 2011;31(1):27‐34. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
KDIGO 2009
Kumar 2017
-
- Kumar J, Bridson JM, Sharma A, Halawa A. Systematic review on role of mammalian target of rapamycin inhibitors as an alternative to calcineurin inhibitors in renal transplant: Challenges and Window to Excel. Experimental & Clinical Transplantation 2017;15(3):241‐52. [MEDLINE: ] - PubMed
Mallet 2017
-
- Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor‐based regimen versus a CNI‐based regimen: a systematic review and meta‐analysis of randomized, controlled trials. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(8):1321–36. [MEDLINE: ] - PMC - PubMed
NHS Blood and Transplant 2019
-
- NHS Blood and Transplant. Organ utilisation evidence. www.odt.nhs.uk/transplantation/organ‐utilisation‐evidence/ (accessed 24 July 2019).
Ponticelli 2004
-
- Ponticelli C. The pleiotropic effects of mTor inhibitors. Journal of Nephrology 2004;17(6):762‐8. [MEDLINE: ] - PubMed
Saunders 2001
-
- Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney International 2001;59(1):3‐16. [MEDLINE: ] - PubMed
Schunemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schuurman 1997
-
- Schuurman HJ, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, et al. SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation 1997;64(1):32‐5. [MEDLINE: ] - PubMed
Stallone 2005
-
- Stallone G, Schena A, Infante B, Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi's sarcoma in renal‐transplant recipients. New England Journal of Medicine 2005;352(13):1317‐23. [MEDLINE: ] - PubMed
Stepkowski 1997
USRDS 2018
-
- United Stated Renal Data System. 2018 Annual Data Report. www.usrds.org/adr.aspx (accessed 24 July 2019).
Viklicky 2000
-
- Viklicky O, Zou H, Muller V, Lacha J, Szabo A, Heemann U. SDZ‐RAD prevents manifestation of chronic rejection in rat renal allografts. Transplantation 2000;69(4):497‐502. [MEDLINE: ] - PubMed
Ying 2018
-
- Ying T, Wong G, Lim W, Kanellis J, Pilmore H, Campbell S, et al. De novo or early conversion to everolimus and long‐term cancer outcomes in kidney transplant recipients: a trial‐based linkage study. American Journal of Transplantation 2018;18(12):2977–86. [MEDLINE: ] - PubMed
References to other published versions of this review
Webster 2003
Webster 2006a
Webster 2006b
-
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta‐analysis of randomized trials. Transplantation 2006;81(9):1234‐48. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

