Polyaromatic hydrocarbons in pollution: a heart-breaking matter
- PMID: 31840250
- PMCID: PMC7003748
- DOI: 10.1113/JP278885
Polyaromatic hydrocarbons in pollution: a heart-breaking matter
Abstract
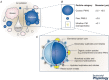
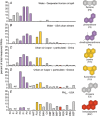
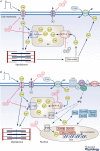
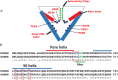
Air pollution is associated with detrimental effects on human health, including decreased cardiovascular function. However, the causative mechanisms behind these effects have yet to be fully elucidated. Here we review the current epidemiological, clinical and experimental evidence linking pollution with cardiovascular dysfunction. Our focus is on particulate matter (PM) and the associated low molecular weight polycyclic aromatic hydrocarbons (PAHs) as key mediators of cardiotoxicity. We begin by reviewing the growing epidemiological evidence linking air pollution to cardiovascular dysfunction in humans. We next address the pollution-based cardiotoxic mechanisms first identified in fish following the release of large quantities of PAHs into the marine environment from point oil spills (e.g. Deepwater Horizon). We finish by discussing the current state of mechanistic knowledge linking PM and PAH exposure to mammalian cardiovascular patho-physiologies such as atherosclerosis, cardiac hypertrophy, arrhythmias, contractile dysfunction and the underlying alterations in gene regulation. Our aim is to show conservation of toxicant pathways and cellular targets across vertebrate hearts to allow a broad framework of the global problem of cardiotoxic pollution to be established. AhR; Aryl hydrocarbon receptor. Dark lines indicate topics discussed in this review. Grey lines indicate topics reviewed elsewhere.
Keywords: PAH; PM; PM2.5; air pollution; cardiotoxicity; cardiovascular dysfunction; heart disease; oil spills; particulate matter; phenanthrene.
© 2019 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures

References
-
- Abdel‐Shafy HI & Mansour MSM (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt J Pet 25, 107–123.
-
- Abramochkin D V., Hassinen M & Vornanen M (2018). Transcripts of Kv7.1 and MinK channels and slow delayed rectifier K+ current (I Ks) are expressed in zebrafish (Danio rerio) heart. Pflugers Arch 470, 1753–1764. - PubMed
-
- European Environment Agency (2019). Air Quality in Europe — 2018 Report Available at: http://www.eea.europa.eu/publications/air-quality-in-europe-2012.
-
- Alday A, Alonso H, Gallego M, Urrutia J, Letamendia A, Callol C & Casis O (2014). Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol Res 84, 26–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

