Murine maternal dietary restriction affects neural Humanin expression and cellular profile
- PMID: 31840315
- PMCID: PMC7744116
- DOI: 10.1002/jnr.24568
Murine maternal dietary restriction affects neural Humanin expression and cellular profile
Abstract
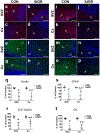
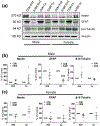
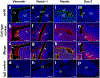
To understand the cellular basis for the neurodevelopmental effects of intrauterine growth restriction (IUGR), we examined the global and regional expression of various cell types within murine (Mus musculus) fetal brain. Our model employed maternal calorie restriction to 50% daily food intake from gestation day 10-19, producing IUGR offspring. Offspring had smaller head sizes with larger head:body ratios indicating a head sparing IUGR effect. IUGR fetuses at embryonic day 19 (E19) had reduced nestin (progenitors), β-III tubulin (immature neurons), Glial fibrillary acidic protein (astrocytes), and O4 (oligodendrocytes) cell lineages via immunofluorescence quantification and a 30% reduction in cortical thickness. No difference was found in Bcl-2 or Bax (apoptosis) between controls and IUGR, though qualitatively, immunoreactivity of doublecortin (migration) and Ki67 (proliferation) was decreased. In the interest of examining a potential therapeutic peptide, we next investigated a novel pro-survival peptide, mouse Humanin (mHN). Ontogeny examination revealed highest mHN expression at E19, diminishing by postnatal day 15 (P15), and nearly absent in adult (3 months). Subanalysis by sex at E19 yielded higher mHN expression among males during fetal life, without significant difference between sexes postnatally. Furthermore, female IUGR mice at E19 had a greater increase in cortical mHN versus the male fetus over their respective controls. We conclude that maternal dietary restriction-associated IUGR interferes with neural progenitors differentiating into the various cellular components populating the cerebral cortex, and reduces cerebral cortical size. mHN expression is developmental stage and sex specific, with IUGR, particularly in the females, adaptively increasing its expression toward mediating a pro-survival approach against nutritional adversity.
Keywords: Humanin; RRID:AB_10695870; RRID:AB_10950489; RRID:AB_1616567; RRID:AB_1619688; RRID:AB_2286684; RRID:AB_2313584; RRID:AB_2313773; RRID:AB_2336177; RRID:AB_2340375; RRID:AB_2340432; RRID:AB_305670; RRID:AB_443209; RRID:AB_477629; RRID:AB_477662; RRID:AB_532250; RRID:AB_561053; RRID:AB_631729; RRID:AB_650336; RRID:AB_732011; RRID:AB_772206; RRID:AB_793998; RRID:SCR_002140; RRID:SCR_002368; RRID:SCR_002798; RRID:SCR_013673; RRID:SCR_014246; RRID:SCR_017411; fetal brain; intrauterine growth restriction; neurodevelopment; nutrient restriction.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Figures





Similar articles
-
Adaptive responses to maternal nutrient restriction alter placental transport in ewes.Placenta. 2020 Jul;96:1-9. doi: 10.1016/j.placenta.2020.05.002. Epub 2020 May 7. Placenta. 2020. PMID: 32421527
-
Quantitative proteomic profiling of the rat substantia nigra places glial fibrillary acidic protein at the hub of proteins dysregulated during aging: Implications for idiopathic Parkinson's disease.J Neurosci Res. 2020 Jul;98(7):1417-1432. doi: 10.1002/jnr.24622. Epub 2020 Apr 9. J Neurosci Res. 2020. PMID: 32270889
-
Maternal food restriction-induced intrauterine growth restriction in a rat model leads to sex-specific adipogenic programming.FASEB J. 2020 Dec;34(12):16073-16085. doi: 10.1096/fj.202000985RR. Epub 2020 Oct 13. FASEB J. 2020. PMID: 33047380 Free PMC article.
-
Thrombopoietin exerts a neuroprotective effect by inhibiting the suppression of neuronal proliferation and axonal outgrowth in intrauterine growth restriction rats.Exp Neurol. 2024 Jul;377:114781. doi: 10.1016/j.expneurol.2024.114781. Epub 2024 Apr 16. Exp Neurol. 2024. PMID: 38636773
-
Biological mechanisms for nutritional regulation of maternal health and fetal development.Paediatr Perinat Epidemiol. 2012 Jul;26 Suppl 1:4-26. doi: 10.1111/j.1365-3016.2012.01291.x. Paediatr Perinat Epidemiol. 2012. PMID: 22742599 Review.
Cited by
-
Adult glut3 homozygous null mice survive to demonstrate neural excitability and altered neurobehavioral responses reminiscent of neurodevelopmental disorders.Exp Neurol. 2021 Apr;338:113603. doi: 10.1016/j.expneurol.2021.113603. Epub 2021 Jan 19. Exp Neurol. 2021. PMID: 33482226 Free PMC article.
-
Sex-dependent effects of prenatal food and protein restriction on offspring physiology in rats and mice: systematic review and meta-analyses.Biol Sex Differ. 2021 Feb 9;12(1):21. doi: 10.1186/s13293-021-00365-4. Biol Sex Differ. 2021. PMID: 33563335 Free PMC article.
-
The Acute Hepatic NF-κB-Mediated Proinflammatory Response to Endotoxemia Is Attenuated in Intrauterine Growth-Restricted Newborn Mice.Front Immunol. 2021 Sep 1;12:706774. doi: 10.3389/fimmu.2021.706774. eCollection 2021. Front Immunol. 2021. PMID: 34539638 Free PMC article.
-
Neurodevelopment Is Dependent on Maternal Diet: Placenta and Brain Glucose Transporters GLUT1 and GLUT3.Nutrients. 2024 Jul 21;16(14):2363. doi: 10.3390/nu16142363. Nutrients. 2024. PMID: 39064806 Free PMC article. Review.
-
Developing Brain Glucose Transporters, Serotonin, Serotonin Transporter, and Oxytocin Receptor Expression in Response to Early-Life Hypocaloric and Hypercaloric Dietary, and Air Pollutant Exposures.Dev Neurosci. 2021;43(1):27-42. doi: 10.1159/000514709. Epub 2021 Mar 26. Dev Neurosci. 2021. PMID: 33774619 Free PMC article.
References
-
- Artavanis-Tsakonas S, Rand MD, & Lake RJ (1999). Notch signaling: cell fate control and signal integration in development. Science, 284(5415), 770–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

