The excitable signal transduction networks: movers and shapers of eukaryotic cell migration
- PMID: 31840779
- PMCID: PMC6956983
- DOI: 10.1387/ijdb.190265pd
The excitable signal transduction networks: movers and shapers of eukaryotic cell migration
Abstract
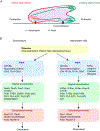
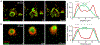
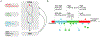
In response to a variety of external cues, eukaryotic cells display varied migratory modes to perform their physiological functions during development and in the adult. Aberrations in cell migration result in embryonic defects and cancer metastasis. The molecular components involved in cell migration are remarkably conserved between the social amoeba Dictyostelium and mammalian cells. This makes the amoeba an excellent model system for studies of eukaryotic cell migration. These migration-associated components can be grouped into three networks: input, signal transduction and cytoskeletal. In migrating cells, signal transduction events such as Ras or PI3K activity occur at the protrusion tips, referred to as 'front', whereas events such as dissociation of PTEN from these regions are referred to as 'back'. Asymmetric distribution of such front and back events is crucial for establishing polarity and guiding cell migration. The triggering of these signaling events displays properties of biochemical excitability including all-or-nothing responsiveness to suprathreshold stimuli, refractoriness, and wave propagation. These signal transduction waves originate from a point and propagate towards the edge of the cell, thereby driving cytoskeletal activity and cellular protrusions. Any change in the threshold for network activation alters the range of the propagating waves and the size of cellular protrusions which gives rise to various migratory modes in cells. Thus, this review highlights excitable signal transduction networks as key players for coordinating cytoskeletal activities to drive cell migration in all eukaryotes.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
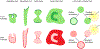
References
-
- Anderson KI and Cross R (2000). “Contact dynamics during keratocyte motility.” Curr Biol 10(5): 253–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

