Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms
- PMID: 31841134
- PMCID: PMC7053580
- DOI: 10.1093/femsre/fuz030
Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms
Abstract
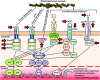
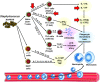
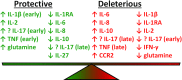
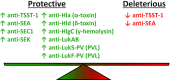
Invasive Staphylococcus aureus infections are a leading cause of morbidity and mortality in both hospital and community settings, especially with the widespread emergence of virulent and multi-drug resistant methicillin-resistant S. aureus strains. There is an urgent and unmet clinical need for non-antibiotic immune-based approaches to treat these infections as the increasing antibiotic resistance is creating a serious threat to public health. However, all vaccination attempts aimed at preventing S. aureus invasive infections have failed in human trials, especially all vaccines aimed at generating high titers of opsonic antibodies against S. aureus surface antigens to facilitate antibody-mediated bacterial clearance. In this review, we summarize the data from humans regarding the immune responses that protect against invasive S. aureus infections as well as host genetic factors and bacterial evasion mechanisms, which are important to consider for the future development of effective and successful vaccines and immunotherapies against invasive S. aureus infections in humans. The evidence presented form the basis for a hypothesis that staphylococcal toxins (including superantigens and pore-forming toxins) are important virulence factors, and targeting the neutralization of these toxins are more likely to provide a therapeutic benefit in contrast to prior vaccine attempts to generate antibodies to facilitate opsonophagocytosis.
Keywords: Staphylococcus aureus; MRSA; evasion; genetics; immunity; vaccine.
© FEMS 2019.
Figures

References
-
- Abdel-Haq N, Al-Tatari H, Chearskul Pet al. .. Methicillin-resistant Staphylococcus aureus (MRSA) in hospitalized children: correlation of molecular analysis with clinical presentation and antibiotic susceptibility testing (ABST) results. Eur J Clin Microbiol Infect Dis. 2009;28:547–51. - PubMed
-
- Adhikari RP, Ajao AO, Aman MJet al. .. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. - PubMed
-
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer Ket al. .. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7. - PubMed
-
- Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2018;53:S64–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

