Pre-Existing Comorbid Emotional Symptoms Moderate Short-Term Methylphenidate Adverse Effects in a Randomized Trial of Children with Attention-Deficit/Hyperactivity Disorder
- PMID: 31841646
- PMCID: PMC7153644
- DOI: 10.1089/cap.2019.0125
Pre-Existing Comorbid Emotional Symptoms Moderate Short-Term Methylphenidate Adverse Effects in a Randomized Trial of Children with Attention-Deficit/Hyperactivity Disorder
Abstract
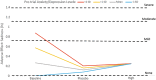
Objective: We sought to ascertain whether baseline anxiety/depression and oppositional defiant disorder (ODD) symptoms impacted the experience of short-term methylphenidate (MPH) adverse effects (AEs) in 7- to 11-year-old children with attention-deficit/hyperactivity disorder (ADHD) (n = 171) undergoing a double-blind MPH crossover trial. Method: The Vanderbilt ADHD Diagnostic Parent Rating Scale measured baseline child anxiety/depression and ODD symptomology. The parent-completed Pittsburgh Side Effect Rating Scale assessed the AEs of anxiety, sadness, and irritability at baseline, on placebo, and on three MPH dosages. For each AE, we evaluated comorbidity main effects, dose main effects, and comorbidity × dose interactions. Results: Baseline anxiety/depression × dose and ODD × dose interactions were significant for the AEs of anxiety, sadness, and irritability. Compared with premedication baseline, these AEs attenuated on MPH for children with initially higher comorbidity symptoms, whereas those with initially lower comorbidity symptoms tended toward no change or increasing AE levels. Conclusion: Premedication anxiety/depressive and ODD symptoms may be important predictors of short-term MPH emotional AEs.
Keywords: ADHD; adverse effects; methylphenidate; side effects; stimulants.
Conflict of interest statement
Dr. Epstein has received research support from Akili Interactive Labs, received royalties from Multi-Health Systems, Inc., received consulting fees from the American Academy of Pediatrics and American Board of Pediatrics, and received licensing fees from Optimal Medicine, Inc. and IXICO. Dr. Froehlich, Dr. Brinkman, Dr. Peugh, Ms. Piedra, and Mr. Vitucci have no conflicts of interest or financial disclosures.
Figures


References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994
-
- Barkley RA: Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment (3rd ed). New York (NY), The Guilford Press, 2006
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

