Monitoring of efficacy, tolerability and safety of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Lambaréné, Gabon: an open-label clinical trial
- PMID: 31842893
- PMCID: PMC6916217
- DOI: 10.1186/s12936-019-3015-4
Monitoring of efficacy, tolerability and safety of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Lambaréné, Gabon: an open-label clinical trial
Abstract
Background: Malaria remains a major public health problem, affecting mainly low-and middle-income countries. The management of this parasitic disease is challenged by ever increasing drug resistance. This study, investigated the therapeutic efficacy, tolerability and safety of artemether-lumefantrine (AL) and artesunate-amodiaquine (AS-AQ), used as first-line drugs to treat uncomplicated malaria in Lambaréné, Gabon.
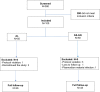
Methods: A non-randomized clinical trial was conducted between October 2017 and March 2018 to assess safety, clinical and parasitological efficacy of fixed-doses of AL and AS-AQ administered to treat uncomplicated Plasmodium falciparum malaria in children aged from 6 months to 12 years. After 50 children were treated with AL, another 50 children received ASAQ. The 2009 World Health Organization protocol for monitoring of the efficacy of anti‑malarial drugs was followed. Molecular markers msp1 and msp2 were used to differentiate recrudescence and reinfection. For the investigation of artemisinin resistant markers, gene mutations in Pfk13 were screened.
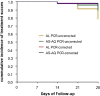
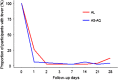
Results: Per-protocol analysis on day 28 showed a PCR corrected cure rate of 97% (95% CI 86-100) and 95% (95% CI 84-99) for AL and AS-AQ, respectively. The most frequent adverse event in both groups was asthenia. No mutations in the kelch-13 gene associated with artemisinin resistance were identified. All participants had completed microscopic parasite clearance by day 3 post-treatment.
Conclusion: This study showed that AL and AS-AQ remain efficacious, well-tolerated, and are safe to treat uncomplicated malaria in children from Lambaréné. However, a regular monitoring of efficacy and a study of molecular markers of drug resistance to artemisinin in field isolates is essential. Trial registration ANZCTR, ACTRN12616001600437. Registered 18 November, http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=ACTRN12616001600437p&isBasic=True.
Keywords: Artemether–lumefantrine; Artesunate–amodiaquine; Efficacy; Gabon; Lambaréné; Malaria; Safety; Tolerability.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization (WHO). World malaria report 2018. Geneva: World Health Organisation; 2018. http://www.who.int/malaria/en/. Accessed 27 Jan 2019.
-
- WHO. Gabon, country profile. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/country-profiles/profile_gab_en.... Accessed 17 Apr 2019.
-
- WHO. Antimalarial drug combination therapy report of a WHO technical consultation. Geneva: World Health Organization; 2001. https://apps.who.int/iris/bitstream/handle/10665/66952/WHO_CDS_RBM_2001?.... Accessed 27 Jan 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials