N-methyl-D-aspartate (NMDA) receptor expression and function is required for early chondrogenesis
- PMID: 31842918
- PMCID: PMC6915923
- DOI: 10.1186/s12964-019-0487-3
N-methyl-D-aspartate (NMDA) receptor expression and function is required for early chondrogenesis
Abstract
Background: In vitro chondrogenesis depends on the concerted action of numerous signalling pathways, many of which are sensitive to the changes of intracellular Ca2+ concentration. N-methyl-D-aspartate (NMDA) glutamate receptor is a cation channel with high permeability for Ca2+. Whilst there is now accumulating evidence for the expression and function of NMDA receptors in non-neural tissues including mature cartilage and bone, the contribution of glutamate signalling to the regulation of chondrogenesis is yet to be elucidated.
Methods: We studied the role of glutamatergic signalling during the course of in vitro chondrogenesis in high density chondrifying cell cultures using single cell fluorescent calcium imaging, patch clamp, transient gene silencing, and western blotting.
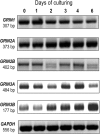
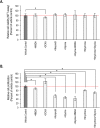
Results: Here we show that key components of the glutamatergic signalling pathways are functional during in vitro chondrogenesis in a primary chicken chondrogenic model system. We also present the full glutamate receptor subunit mRNA and protein expression profile of these cultures. This is the first study to report that NMDA-mediated signalling may act as a key factor in embryonic limb bud-derived chondrogenic cultures as it evokes intracellular Ca2+ transients, which are abolished by the GluN2B subunit-specific inhibitor ifenprodil. The function of NMDARs is essential for chondrogenesis as their functional knock-down using either ifenprodil or GRIN1 siRNA temporarily blocks the differentiation of chondroprogenitor cells. Cartilage formation was fully restored with the re-expression of the GluN1 protein.
Conclusions: We propose a key role for NMDARs during the transition of chondroprogenitor cells to cartilage matrix-producing chondroblasts.
Keywords: Chondrocyte; Chondrogenesis; Glutamate signalling; Glycine; N-methyl-D-aspartate receptor; NMDAR; Single cell calcium imaging; siRNA.
Conflict of interest statement
The authors declare that they have no competing interests. This paper was written by the authors within the scope of their academic and research positions. None of the authors have any relationships that could be construed as biased or inappropriate. The funding bodies were not involved in the study design, data collection, analysis and interpretation. The decision to submit the paper for publication was not influenced by any the funding bodies.
Figures






References
-
- Varga Z, Juhasz T, Matta C, Fodor J, Katona E, Bartok A, et al. Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2+−oscillations and cartilage formation. PLoS One. 2011;6(11):e27957. doi: 10.1371/journal.pone.0027957. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

