Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review
- PMID: 31842957
- PMCID: PMC6916124
- DOI: 10.1186/s13058-019-1210-4
Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review
Abstract
Background: Metastatic triple-negative breast cancer (mTNBC), an aggressive histological subtype, has poor prognosis. Chemotherapy remains standard of care for mTNBC, although no agent has been specifically approved for this breast cancer subtype. Instead, chemotherapies approved for metastatic breast cancer (MBC) are used for mTNBC (National Comprehensive Cancer Network Guidelines [NCCN] v1.2019). Atezolizumab in combination with nab-paclitaxel was recently approved for programmed death-ligand 1 (PD-L1)-positive locally advanced or metastatic TNBC. Published historical data were reviewed to characterize the efficacy of NCCN-recommended (v1.2016) agents as first-line (1L) and second-line or later (2L+) treatment for patients with locally recurrent inoperable or metastatic TNBC (collectively termed mTNBC herein).
Methods: A systematic literature review was performed, examining clinical efficacy of therapies for mTNBC based on NCCN v1.2016 guideline recommendations. Data from 13 studies, either published retrospective mTNBC subgroup analyses based on phase III trials in MBC or phase II trials in mTNBC, were included.
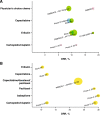
Results: A meta-analysis of mTNBC subgroups from three phase III trials in 1L MBC reported pooled objective response rate (ORR) of 23%, median overall survival (OS) of 17.5 months, and median progression-free survival (PFS) of 5.4 months with single-agent chemotherapy. In two subgroup analyses from a phase III study and a phase II trial (n = 40 each), median duration of response (DOR) to 1L chemotherapy for mTNBC was 4.4-6.6 months; therefore, responses were not durable. A meta-analysis of seven cohorts showed the pooled ORR for 2L+ chemotherapy was 11% (95% CI, 9-14%). Median DOR to 2L+ chemotherapy in mTNBC was also limited (4.2-5.9 months) per two subgroup analyses from a phase III study. No combination chemotherapy regimens recommended by NCCN v1.2016 for treatment of MBC showed superior OS to single agents.
Conclusions: Chemotherapies have limited effectiveness and are associated with unfavorable toxicity profiles, highlighting a considerable unmet medical need for improved therapeutic options in mTNBC. In addition to the recently approved combination of atezolizumab and nab-paclitaxel for PD-L1-positive mTNBC, new treatments resulting in durable clinical responses, prolonged survival, and manageable safety profile would greatly benefit patients with mTNBC.
Keywords: Chemotherapy; Immune checkpoint inhibitor; Metastatic triple-negative breast cancer; PARP inhibitor.
Conflict of interest statement
CHL, VK, GA, and ML are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Figures


References
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. - DOI - PubMed
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): breast cancer (Version 1.2019). Accessed 8 July 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

