Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis
- PMID: 31843251
- PMCID: PMC7681916
- DOI: 10.1016/j.jvsv.2019.03.023
Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis
Abstract
Background: After deep venous thrombosis (DVT), many patients have impaired quality of life (QOL). We aimed to assess whether pharmacomechanical catheter-directed thrombolysis (PCDT) improves short-term or long-term QOL in patients with proximal DVT and whether QOL is related to extent of DVT.
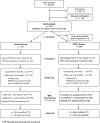
Methods: The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial was an assessor-blinded randomized trial that compared PCDT with no PCDT in patients with DVT of the femoral, common femoral, or iliac veins. QOL was assessed at baseline and 1 month, 6 months, 12 months, 18 months, and 24 months using the Venous Insufficiency Epidemiological and Economic Study on Quality of Life/Symptoms (VEINES-QOL/Sym) disease-specific QOL measure and the 36-Item Short Form Health Survey (SF-36) physical component summary (PCS) and mental component summary general QOL measures. Change in QOL scores from baseline to assessment time were compared in the PCDT and no PCDT treatment groups overall and in the iliofemoral DVT and femoral-popliteal DVT subgroups.
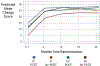
Results: Of 692 ATTRACT patients, 691 were analyzed (mean age, 53 years; 62% male; 57% iliofemoral DVT). VEINES-QOL change scores were greater (ie, better) in PCDT vs no PCDT from baseline to 1 month (difference, 5.7; P = .0006) and from baseline to 6 months (5.1; P = .0029) but not for other intervals. SF-36 PCS change scores were greater in PCDT vs no PCDT from baseline to 1 month (difference, 2.4; P = .01) but not for other intervals. Among iliofemoral DVT patients, VEINES-QOL change scores from baseline to all assessments were greater in the PCDT vs no PCDT group; this was statistically significant in the intention-to-treat analysis at 1 month (difference, 10.0; P < .0001) and 6 months (8.8; P < .0001) and in the per-protocol analysis at 18 months (difference, 5.8; P = .0086) and 24 months (difference, 6.6; P = .0067). SF-36 PCS change scores were greater in PCDT vs no PCDT from baseline to 1 month (difference, 3.2; P = .0010) but not for other intervals. In contrast, in femoral-popliteal DVT patients, change scores from baseline to all assessments were similar in the PCDT and no PCDT groups.
Conclusions: Among patients with proximal DVT, PCDT leads to greater improvement in disease-specific QOL than no PCDT at 1 month and 6 months but not later. In patients with iliofemoral DVT, PCDT led to greater improvement in disease-specific QOL during 24 months.
Trial registration: ClinicalTrials.gov NCT00790335.
Keywords: Catheter-directed thrombolysis; Deep venous thrombosis; Femoral-popliteal DVT; Iliofemoral DVT; Proximal DVT; Quality of life; Randomized trial.
Copyright © 2019 Society for Vascular Surgery. All rights reserved.
Figures

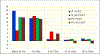
Comment in
-
The future of iliofemoral deep vein thrombosis treatment.J Vasc Surg Venous Lymphat Disord. 2019 Nov;7(6):771-772. doi: 10.1016/j.jvsv.2019.07.002. J Vasc Surg Venous Lymphat Disord. 2019. PMID: 31627872 No abstract available.
References
-
- Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2014;130(18):1636–61. - PubMed
-
- van Korlaar I, Vossen C, Rosendaal F, Cameron L, Bovill E, Kaptein A. Quality of life in venous disease. Thromb Haemost. 2003;90(1):27–35. - PubMed
-
- Kahn SR, Ducruet T, Lamping DL, Arsenault L, Miron MJ, Roussin A, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165(10):1173–8. - PubMed
-
- Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

