Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma
- PMID: 31843922
- PMCID: PMC6936349
- DOI: 10.1073/pnas.1914915116
Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma
Erratum in
-
Correction for Porter et al., Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma.Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1818. doi: 10.1073/pnas.1922469117. Epub 2020 Jan 13. Proc Natl Acad Sci U S A. 2020. PMID: 31932438 Free PMC article. No abstract available.
Abstract
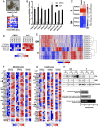
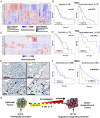
Transcriptional profiling has defined pancreatic ductal adenocarcinoma (PDAC) into distinct subtypes with the majority being classical epithelial (E) or quasi-mesenchymal (QM). Despite clear differences in clinical behavior, growing evidence indicates these subtypes exist on a continuum with features of both subtypes present and suggestive of interconverting cell states. Here, we investigated the impact of different therapies being evaluated in PDAC on the phenotypic spectrum of the E/QM state. We demonstrate using RNA-sequencing and RNA-in situ hybridization (RNA-ISH) that FOLFIRINOX combination chemotherapy induces a common shift of both E and QM PDAC toward a more QM state in cell lines and patient tumors. In contrast, Vitamin D, another drug under clinical investigation in PDAC, induces distinct transcriptional responses in each PDAC subtype, with augmentation of the baseline E and QM state. Importantly, this translates to functional changes that increase metastatic propensity in QM PDAC, but decrease dissemination in E PDAC in vivo models. These data exemplify the importance of both the initial E/QM subtype and the plasticity of E/QM states in PDAC in influencing response to therapy, which highlights their relevance in guiding clinical trials.
Keywords: Vitamin D; molecular subtypes; pancreatic ductal adenocarcinoma.
Conflict of interest statement
Competing interest statement: D.T.T. has received consulting fees from EMD Millipore-Sigma, Ventana-Roche, Foundation Medicine, Inc., and Merrimack Pharmaceuticals. D.T.T. is a founder and has equity in PanTher Therapeutics, ROME Therapeutics, and TellBio Inc., which are not related to this work. D.A.H. is founder and has equity in TellBio Inc., which is not related to this work. D.T.T.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. D.T.T. and V.D. receive sponsored research support from ACD-Biotechne that was performed for some parts of this work. V.D. receives sponsored research support from Agios. M.S.L. and E.A.C. are coauthors on a 2016 paper.
Figures


References
-
- Bailey P., et al. ; Australian Pancreatic Cancer Genome Initiative , Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

