Corazonin signaling integrates energy homeostasis and lunar phase to regulate aspects of growth and sexual maturation in Platynereis
- PMID: 31843923
- PMCID: PMC6969523
- DOI: 10.1073/pnas.1910262116
Corazonin signaling integrates energy homeostasis and lunar phase to regulate aspects of growth and sexual maturation in Platynereis
Abstract
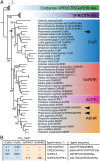
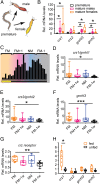
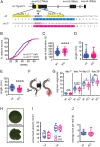
The molecular mechanisms by which animals integrate external stimuli with internal energy balance to regulate major developmental and reproductive events still remain enigmatic. We investigated this aspect in the marine bristleworm, Platynereis dumerilii, a species where sexual maturation is tightly regulated by both metabolic state and lunar cycle. Our specific focus was on ligands and receptors of the gonadotropin-releasing hormone (GnRH) superfamily. Members of this superfamily are key in triggering sexual maturation in vertebrates but also regulate reproductive processes and energy homeostasis in invertebrates. Here we show that 3 of the 4 gnrh-like (gnrhl) preprohormone genes are expressed in specific and distinct neuronal clusters in the Platynereis brain. Moreover, ligand-receptor interaction analyses reveal a single Platynereis corazonin receptor (CrzR) to be activated by CRZ1/GnRHL1, CRZ2/GnRHL2, and GnRHL3 (previously classified as AKH1), whereas 2 AKH-type hormone receptors (GnRHR1/AKHR1 and GnRHR2/AKHR2) respond only to a single ligand (GnRH2/GnRHL4). Crz1/gnrhl1 exhibits a particularly strong up-regulation in sexually mature animals, after feeding, and in specific lunar phases. Homozygous crz1/gnrhl1 knockout animals exhibit a significant delay in maturation, reduced growth, and attenuated regeneration. Through a combination of proteomics and gene expression analysis, we identify enzymes involved in carbohydrate metabolism as transcriptional targets of CRZ1/GnRHL1 signaling. Our data suggest that Platynereis CRZ1/GnRHL1 coordinates glycoprotein turnover and energy homeostasis with growth and sexual maturation, integrating both metabolic and developmental demands with the worm's monthly cycle.
Keywords: GnRH; corazonin; lunar periodicity; regeneration; reproduction.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Evolutionarily conserved peptides coordinate lunar phase and metabolism.Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):805-807. doi: 10.1073/pnas.1920432117. Epub 2019 Dec 30. Proc Natl Acad Sci U S A. 2020. PMID: 31888992 Free PMC article. No abstract available.
References
-
- Colombani J., et al. , A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739–749 (2003). - PubMed
-
- Schultz T. F., Kay S. A., Circadian clocks in daily and seasonal control of development. Science 301, 326–328 (2003). - PubMed
-
- Ebling F. J. P., Barrett P., The regulation of seasonal changes in food intake and body weight. J. Neuroendocrinol. 20, 827–833 (2008). - PubMed
-
- Fenelon J. C., Banerjee A., Murphy B. D., Embryonic diapause: Development on hold. Int. J. Dev. Biol. 58, 163–174 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

