Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson's disease model
- PMID: 31844040
- PMCID: PMC6915731
- DOI: 10.1038/s41419-019-2184-y
Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson's disease model
Abstract
A primary cilium is an antenna-like structure on the cell surface that plays a crucial role in sensory perception and signal transduction. Mitochondria, the 'powerhouse' of the cell, control cell survival, and death. The cellular ability to remove dysfunctional mitochondria through mitophagy is important for cell survival. We show here that mitochondrial stress, caused by respiratory complex inhibitors and excessive fission, robustly stimulates ciliogenesis in different types of cells including neuronal cells. Mitochondrial stress-induced ciliogenesis is mediated by mitochondrial reactive oxygen species generation, subsequent activation of AMP-activated protein kinase and autophagy. Conversely, abrogation of ciliogenesis compromises mitochondrial stress-induced autophagy, leading to enhanced cell death. In mice, treatment with mitochondrial toxin, MPTP elicits ciliary elongation and autophagy in the substantia nigra dopamine neurons. Blockade of cilia formation in these neurons attenuates MPTP-induced autophagy but facilitates dopamine neuronal loss and motor disability. Our findings demonstrate the important role of primary cilia in cellular pro-survival responses during mitochondrial stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



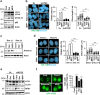
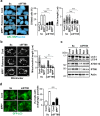
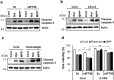
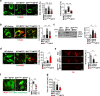
References
Publication types
MeSH terms
Substances
Grants and funding
- 2017R1A2B4005501/National Research Foundation of Korea (NRF)/International
- 2017M3A9G7073521/National Research Foundation of Korea (NRF)/International
- 2017R1A2B3007123/National Research Foundation of Korea (NRF)/International
- 2017-757, 2018-326/Asan Institute for Life Sciences, Asan Medical Center/International
LinkOut - more resources
Full Text Sources
Medical

