Colorectal cancers utilize glutamine as an anaplerotic substrate of the TCA cycle in vivo
- PMID: 31844152
- PMCID: PMC6915720
- DOI: 10.1038/s41598-019-55718-2
Colorectal cancers utilize glutamine as an anaplerotic substrate of the TCA cycle in vivo
Abstract
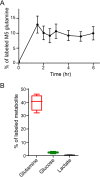
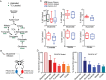
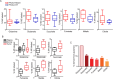
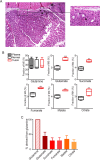
Cancer cells in culture rely on glutamine as an anaplerotic substrate to replenish tricarboxylic acid (TCA) cycle intermediates that have been consumed. but it is uncertain whether cancers in vivo depend on glutamine for anaplerosis. Here, following in vivo infusions of [13C5]-glutamine in mice bearing subcutaneous colon cancer xenografts, we showed substantial amounts of infused [13C5]-glutamine enters the TCA cycle in the tumors. Consistent with our prior observation that colorectal cancers (CRCs) with oncogenic mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic (PIK3CA) subunit are more dependent on glutamine than CRCs with wild type PIK3CA, labeling from glutamine to most TCA cycle intermediates was higher in PIK3CA-mutant subcutaneous xenograft tumors than in wild type PIK3CA tumors. Moreover, using orthotopic mouse colon tumors estalished from human CRC cells or patient-derived xenografts, we demonstrated substantial amounts of infused [13C5]-glutamine enters the TCA cycle in the tumors and tumors utilize anaplerotic glutamine to a greater extent than adjacent normal colon tissues. Similar results were seen in spontaneous colon tumors arising in genetically engineered mice. Our studies provide compelling evidence CRCs utilizes glutamine to replenish the TCA cycle in vivo, suggesting that targeting glutamine metabolism could be a therapeutic approach for CRCs, especially for PIK3CA-mutant CRCs.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer.Nat Commun. 2016 Jun 20;7:11971. doi: 10.1038/ncomms11971. Nat Commun. 2016. PMID: 27321283 Free PMC article.
-
Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner.Nat Commun. 2018 Feb 7;9(1):545. doi: 10.1038/s41467-018-02951-4. Nat Commun. 2018. PMID: 29416026 Free PMC article.
-
5-Fluorouracil Enhances the Antitumor Activity of the Glutaminase Inhibitor CB-839 against PIK3CA-Mutant Colorectal Cancers.Cancer Res. 2020 Nov 1;80(21):4815-4827. doi: 10.1158/0008-5472.CAN-20-0600. Epub 2020 Sep 9. Cancer Res. 2020. PMID: 32907836 Free PMC article. Clinical Trial.
-
Glutamine: an anaplerotic precursor.Nutrition. 2002 Mar;18(3):222-4. doi: 10.1016/s0899-9007(01)00795-x. Nutrition. 2002. PMID: 11882393 Review.
-
Interaction between glutamine availability and metabolism of glycogen, tricarboxylic acid cycle intermediates and glutathione.J Nutr. 2001 Sep;131(9 Suppl):2488S-90S; discussion 2496S-7S. doi: 10.1093/jn/131.9.2488S. J Nutr. 2001. PMID: 11533298 Review.
Cited by
-
The functional roles of TCA cycle metabolites in cancer.Oncogene. 2021 May;40(19):3351-3363. doi: 10.1038/s41388-020-01639-8. Epub 2021 Apr 16. Oncogene. 2021. PMID: 33864000 Review.
-
Glutaminase Inhibitors Induce Thiol-Mediated Oxidative Stress and Radiosensitization in Treatment-Resistant Cervical Cancers.Mol Cancer Ther. 2020 Dec;19(12):2465-2475. doi: 10.1158/1535-7163.MCT-20-0271. Epub 2020 Oct 21. Mol Cancer Ther. 2020. PMID: 33087507 Free PMC article.
-
Hypoxia-induced cysteine metabolism reprogramming is crucial for the tumorigenesis of colorectal cancer.Redox Biol. 2024 Sep;75:103286. doi: 10.1016/j.redox.2024.103286. Epub 2024 Jul 26. Redox Biol. 2024. PMID: 39079386 Free PMC article.
-
Mitochondrial complex IV defects induce metabolic and signaling perturbations that expose potential vulnerabilities in HCT116 cells.FEBS Open Bio. 2022 May;12(5):959-982. doi: 10.1002/2211-5463.13398. Epub 2022 Apr 1. FEBS Open Bio. 2022. PMID: 35302710 Free PMC article.
-
Metabolic Effects of the Cancer Metastasis Modulator MEMO1.Metabolites. 2025 Apr 17;15(4):277. doi: 10.3390/metabo15040277. Metabolites. 2025. PMID: 40278406 Free PMC article.
References
-
- Earle WR, Evans VJ, Hawkins NM, Peppers EV, Westfall BB. Effect of glutamine on the growth and metabolism of liver cells in vitro. Journal of the National Cancer Institute. 1956;17:131–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

