Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation
- PMID: 31844155
- PMCID: PMC7080209
- DOI: 10.1038/s41551-019-0485-1
Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation
Erratum in
-
Author Correction: Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation.Nat Biomed Eng. 2021 Aug;5(8):944-945. doi: 10.1038/s41551-021-00725-w. Nat Biomed Eng. 2021. PMID: 34131325
Abstract
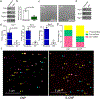
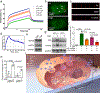
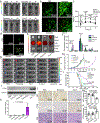
Exosomes are attractive as nucleic-acid carriers because of their favourable pharmacokinetic and immunological properties and their ability to penetrate physiological barriers that are impermeable to synthetic drug-delivery vehicles. However, inserting exogenous nucleic acids, especially large messenger RNAs, into cell-secreted exosomes leads to low yields. Here we report a cellular-nanoporation method for the production of large quantities of exosomes containing therapeutic mRNAs and targeting peptides. We transfected various source cells with plasmid DNAs and stimulated the cells with a focal and transient electrical stimulus that promotes the release of exosomes carrying transcribed mRNAs and targeting peptides. Compared with bulk electroporation and other exosome-production strategies, cellular nanoporation produced up to 50-fold more exosomes and a more than 103-fold increase in exosomal mRNA transcripts, even from cells with low basal levels of exosome secretion. In orthotopic phosphatase and tensin homologue (PTEN)-deficient glioma mouse models, mRNA-containing exosomes restored tumour-suppressor function, enhanced inhibition of tumour growth and increased survival. Cellular nanoporation may enable the use of exosomes as a universal nucleic-acid carrier for applications requiring transcriptional manipulation.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures





Comment in
-
Functional extracellular vesicles aplenty.Nat Biomed Eng. 2020 Jan;4(1):9-11. doi: 10.1038/s41551-019-0507-z. Nat Biomed Eng. 2020. PMID: 31937938 No abstract available.
References
-
- Dickson D UK scientists test liposome gene therapy technique. Nature 365, 4 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

