Dwarna: a blockchain solution for dynamic consent in biobanking
- PMID: 31844175
- PMCID: PMC7170942
- DOI: 10.1038/s41431-019-0560-9
Dwarna: a blockchain solution for dynamic consent in biobanking
Abstract
Dynamic consent aims to empower research partners and facilitate active participation in the research process. Used within the context of biobanking, it gives individuals access to information and control to determine how and where their biospecimens and data should be used. We present Dwarna-a web portal for 'dynamic consent' that acts as a hub connecting the different stakeholders of the Malta Biobank: biobank managers, researchers, research partners, and the general public. The portal stores research partners' consent in a blockchain to create an immutable audit trail of research partners' consent changes. Dwarna's structure also presents a solution to the European Union's General Data Protection Regulation's right to erasure-a right that is seemingly incompatible with the blockchain model. Dwarna's transparent structure increases trustworthiness in the biobanking process by giving research partners more control over which research studies they participate in, by facilitating the withdrawal of consent and by making it possible to request that the biospecimen and associated data are destroyed.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

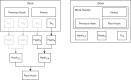

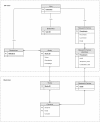
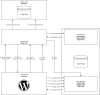
Comment in
-
Detection and protection mechanisms against vulnerabilities are needed in blockchain applications.Eur J Hum Genet. 2020 Jun;28(6):695. doi: 10.1038/s41431-020-0597-9. Epub 2020 Mar 2. Eur J Hum Genet. 2020. PMID: 32123327 Free PMC article. No abstract available.
-
Reply to Y. Takefuji.Eur J Hum Genet. 2020 Jun;28(6):696. doi: 10.1038/s41431-020-0598-8. Epub 2020 Mar 2. Eur J Hum Genet. 2020. PMID: 32123328 Free PMC article. No abstract available.
References
-
- World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. In: 64th WMA General Assembly. 2013. - PubMed
-
- World Health Organization. Council for International Organizations of Medical Sciences. Commentary on Guideline 9. International ethical guidelines for health-related research involving humans. World Health Organization; 2016.
-
- European Union. Article 1—charter of fundamental rights of the European Union. OJ C 202. European Union; 2016. p. 389–405.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

